Published online Aug 25, 2016. doi: 10.4239/wjd.v7.i16.333
Peer-review started: February 17, 2016
First decision: April 15, 2016
Revised: May 8, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: August 25, 2016
Processing time: 188 Days and 15.9 Hours
Diabetic retinopathy (DR) is the leading cause of blindness in industrialized countries. Remarkable advances in the diagnosis and treatment of DR have been made during the past 30 years, but several important management questions and treatment deficiencies remain unanswered. The global diabetes epidemic threatens to overwhelm resources and increase the incidence of blindness, necessitating the development of innovative programs to diagnose and treat patients. The introduction and rapid adoption of intravitreal pharmacologic agents, particularly drugs that block the actions of vascular endothelial growth factor (VEGF) and corticosteroids, have changed the goal of DR treatment from stabilization of vision to improvement. Anti-VEGF injections improve visual acuity in patients with diabetic macular edema (DME) from 8-12 letters and improvements with corticosteroids are only slightly less. Unfortunately, a third of patients have an incomplete response to anti-VEGF therapy, but the best second-line therapy remains unknown. Current first-line therapy requires monthly visits and injections; longer acting therapies are needed to free up healthcare resources and improve patient compliance. VEGF suppression may be as effective as panretinal photocoagulation (PRP) for proliferative diabetic retinopathy, but more studies are needed before PRP is abandoned. For over 30 years laser was the mainstay for the treatment of DME, but recent studies question its role in the pharmacologic era. Aggressive treatment improves vision in most patients, but many still do not achieve reading and driving vision. New drugs are needed to add to gains achieved with available therapies.
Core tip: Newly introduced pharmacotherapies have contributed significantly to the treatment of diabetic retinopathy over the past 10 years and have become first-line therapy. Several questions regarding the best management of certain diabetic conditions remain and new therapies are needed to improve outcomes. Ongoing research and development should address many of these issues over the next 10 years.
- Citation: Stewart MW. Treatment of diabetic retinopathy: Recent advances and unresolved challenges. World J Diabetes 2016; 7(16): 333-341
- URL: https://www.wjgnet.com/1948-9358/full/v7/i16/333.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i16.333
Diabetes mellitus (DM) has become a global health problem fueled by increased caloric consumption and the resultant obesity epidemic[1]. Microvascular complications of DM (retinopathy, nephropathy and neuropathy) are increasingly important causes of morbidity and mortality, and care for affected patients contributes to burgeoning healthcare costs. Diabetic retinopathy (DR) is the leading cause of blindness in working-aged individuals in industrialized nations[2]; diabetic macular edema (DME) accounts for 75% of DR-related vision loss with complications of proliferative diabetic retinopathy (PDR) responsible for most of the balance.
The development, testing, and adoption of advanced intravitreal pharmacotherapy has significantly improved the treatment of diabetic retinopathy over the past decade. The pivotal phase III drug trials demonstrated that binding diffusible vascular endothelial growth factor (VEGF) improves visual acuity (VA) in the majority of patients. Monthly injections of ranibizumab (Lucentis®, Genentech, S. San Francisco, CA, United States/Roche, Basel, Switzerland) and aflibercept (Eylea®, Regeneron, Tarrytown, NY, United States) prevent progression to PDR in many high risk eyes and reverse diabetic retinopathy severity scores in a significant minority[3,4]. Ranibizumab injections are as effective as panretinal photocoagulation at controlling the complications of PDR, while causing fewer visual side effects[5].
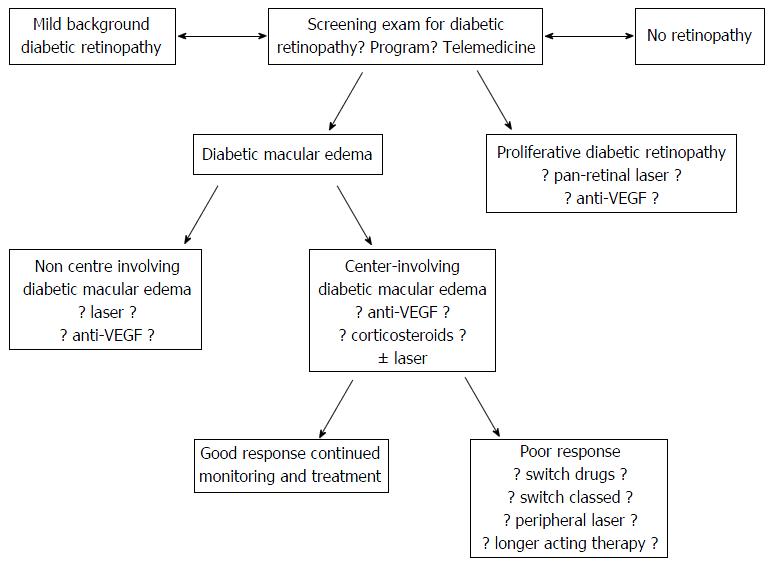
These tremendous advances could not have been imagined by most practitioners 20 years ago, but many questions regarding the optimal therapy for different clinical situations remain unanswered (Figure 1). Additionally, the majority of the world’s population does not have access to specialized care and affordable drugs. This manuscript will discuss some of the unmet challenges pertaining to the treatment of diabetic retinopathy care and will speculate on anticipated solutions.
Effective management of DR depends heavily on early diagnosis and prompt treatment. Laser photocoagulation of DME according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) guidelines for clinically significant macular edema decreases the risk of moderate vision loss (15 letters) by 50% over three years, but far fewer patients experience comparable VA gains[6]. Anti-VEGF therapy, on the other hand, improves VA by a mean of 8-12 ETDRS letters, but eyes with significant acuity loss (< 20/100) usually do not recover reading or driving vision[3,4].
Early diagnoses of DR are made by dilated fundus examinations that are usually performed after patients are referred to ophthalmologists or are seen within a DR screening program. Prominent professional organizations including the American Academy of Pediatrics, the American Academy of Ophthalmology, the American Diabetes Association, and the Canadian Ophthalmological Society have published screening guidelines[7-10]. These generally agree that patients need yearly dilated fundus examinations beginning at the time of diagnosis for patients with type 2 DM and after an interval of 3-5 years for patients with type 1 DM. Despite long-standing efforts to educate patients, primary care physicians, and endocrinologists, only 50% of patients undergo screening eye examinations in any given year and 16% receive exams in two consecutive years[11].
Many industrialized countries have sufficient ophthalmologists to effectively screen their diabetic populations, but non-compliance with screening guidelines is common because of socioeconomic, cultural, and geographic reasons[12]. Patients may be unaware of diabetes-related risks of vision loss and primary care physicians’ referral patterns may be inadequate. Education programs directed at both physicians and patients need improvement, but these are unlikely to completely fix deficiencies in screening[13].
Screening programs are particularly important in developing countries where an insufficient number of physicians and long travel distances preclude the performance of recommended dilated fundus examinations[14]. Telemedicine has the potential to deliver affordable care to many of these underserved populations. Telemedicine DR screening programs can be created with modest up-front purchases of standard or non-mydriatic fundus cameras combined with training of office personnel to take high-quality fundus photographs. Newly developed cellular telephone adapters can turn nearly any telephone into a high-quality fundus camera[15]. The low cost of the adapters together with the widespread availability of cellular telephones allows for placement of a fundus camera in nearly any office. Patients can then receive high quality retinopathy screening evaluations in their primary physicians’ offices. Photos are electronically transmitted to ophthalmologists’ offices or image reading centers for evaluation. Patients with high-risk fundus abnormalities can be referred for ophthalmology examinations or scheduled for future photographs. Issues concerning Health Insurance Portability and Accountability Act compliance and insurance billing can be challenging, but systems that successfully address these concerns have been developed.
Photographs are transmitted via the internet to reading centers staffed with readers. Some centers employ specially trained technicians to read submitted clinical photographs, whereas others use ophthalmologists and retina specialists. As screening programs expand and reach more patients the number of transmitted photographs may overwhelm the ability of reading centers to properly evaluate them. Computer programs are being developed to digitally identify abnormalities on photos, make a diagnosis, assess the risk of vision loss, and recommend referral to an ophthalmologist or defer for future screening[16,17]. Developing and validating software is a complicated task, and powerful hardware is needed to read thousands of photographs, but software developers in several countries are developing programs that may be commercially available within 5 years.
The pivotal phase III anti-VEGF trials evaluated the efficacy of monthly (ranibizumab and aflibercept) or bimonthly (aflibercept) injections on center-involving DME[3,4]. Patients switched to pro re nata (PRN) ranibizumab after 12 mo in RESTORE[18] and after 36 mo in RISE/RIDE[19]. Treating patients according to these strategies probably produces the best possible visual results, but treatment is expensive and compliance is difficult to maintain. Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocols I[20] and T[21] used monthly injections for 4 mo or until dry before switching to monthly PRN protocols based on retreatment criteria that many physicians believe are too complex to use in most clinical settings. Neovascular age-related macular degeneration (nAMD) may be adequately treated with monthly PRN injections after single loading doses[22], but most investigators believe that DME responds slower to therapy and that a prolonged series of initial injections is needed. Monthly treatment regimens, however, conflict with the real-world treatment of DME as most physicians use a treat-and-extend strategy (T and E) regimen for both nAMD and DM[23].
As-needed (PRN) treatment regimens reduce the number of injections, but not the number of clinic visits. The 24-mo, single-masked RETAIN trial compared T and E + laser, T and E, and PRN ranibizumab regimens in patients with DME[24]. Patients in all groups were treated monthly until dry. The VA improvement in patients receiving T and E + laser, T and E, and PRN were similar (+5.9, +6.1, +6.2 letters). The mean numbers of injections were 12.4, 12.8, and 10.7, but patients treated with T and E required 46% fewer clinic visits. Over 70% of patients had treatment intervals extended to at least 2 mo. Though trial design differences make it difficult to directly compare these data to those from the phase III trials, the results are encouraging and will not deter physicians from employing a T and E regimen. A multicenter, randomized trial comparing monthly therapy with T and E is needed, but its cost would likely be prohibitive.
The National Eye Institute funded DRCR.net Protocol T gave us the best comparative data for the three anti-VEGF drugs[21]. For eyes with baseline visual acuities of 20/32-20/40, each of the drugs produced VA gains of +8 letters. But for eyes with baseline VA of 20/50 or worse, aflibercept (+18.9 letters) produced greater gains in VA than ranibizumab (+14.2 letters) and bevacizumab (+11.8 letters). Aflibercept also produced greater macular thinning (-169 μm) than either ranibizumab (-147 μm) or bevacizumab (-101 μm). On average, patients received fewer aflibercept (9) than ranibizumab (10) or bevacizumab (10) injections.
Several issues (compounding of bevacizumab, use of ETDRS visual acuity, complex retreatment criteria) call into question the applicability of the Protocol T data. But many physicians use the results literally; for patients with VA of 20/40 or better they use bevacizumab because of its lower cost; for eyes with VA of 20/50 or worse they use aflibercept because of its greater efficacy[25]. Additional trials are needed to validate these data and identify additional subgroups that respond particularly well or poorly to treatment.
The dexamethasone delivery system (DDS, Ozurdex®, Allergan, Irvine, CA, United States) and the fluocinolone acetonide insert (Iluvien®, Alimera Sciences, Alpharetta, GA, United States) have both been approved for the treatment of DME. The DDS was originally approved for use in pseudophakic eyes or phakic eyes scheduled to undergo cataract removal, but approval for use in phakic eyes followed within months. Unfortunately, neither drug has been directly compared to anti-VEGF therapy in prospective, masked, randomized, multicenter trials. Visual acuity improvements for these sustained delivery systems average +7 letters[26,27], generally less than the +8 to +12 letters achieved with anti-VEGF therapy. Patients in the FAME trial (fluocinolone insert) with DME of > 3 years duration responded better to the insert than did those with non-chronic DME. This suggests that chronic DME is chemokine-driven whereas non-chronic DME is VEGF driven[28]. Perhaps the best treatment strategy is to use anti-VEGF drugs for non-chronic DME and reserve corticosteroid use for chronic DME. Three years of corticosteroid therapy leads to high rates of cataract development (91%) and elevated intraocular pressure (> 30%). For these reasons, intraocular corticosteroids may be effective second-line therapy, but are usually not used as first-line therapy.
Small, single center studies suggest that the DDS produces comparable VA improvements to those of bevacizumab and ranibizumab, and perhaps superior macular thinning, but randomized, multicenter trials are needed to determine relative efficacies.
Monthly anti-VEGF therapy appears to decrease the need for therapy after one year[29] and improves DR severity after two years[3]. By contrast, nAMD appears to require therapy for longer periods of time[30]. Not surprisingly, development of anti-VEGF drugs has been driven primarily by the need for nAMD therapy. Phase III nAMD trials using longer acting anti-VEGF agents such as abicipar pegol, a designed ankyrin repeat protein, and RTH508, a single-strand antibody fragment, are underway. A phase I/II multicenter, dose-escalation trial evaluated the safety and bioactivity of abicipar in 18 patients with DME[31]. Patients in the 1 mg cohort who received single injections experienced excellent reduction in macular thickness and average VA improvements of +10 letters at 12 wk. Pharmacokinetic analyses based on anterior chamber drug concentrations suggested an extended intraocular half-life of 13.4 d. The phase III nAMD trial is hoping to show efficacy with q12week dosing that is comparable to q4week ranibizumab[32]. A phase II DME trial has not yet been announced.
A refillable, trans-scleral ranibizumab reservoir underwent phase I nAMD testing with 20 patients in Latvia[33]. Patients required an average of 4.8 refills of the reservoir (500 μg each time) and achieved VA improvements comparable to those from the pivotal phase III trials. Four of the 20 eyes experienced significant complications due to implantation, requiring a modification of the surgical technique.
An anti-VEGF producing encapsulated cell chamber filled with immortalized retinal pigment epithelial cells is entering phase II trials for nAMD[34]. The genetically modified cells produce a high-affinity, VEGF-binding protein. In a previous trial this technology produced ciliary neurotrophic factor with a cell half-life of 51 mo[35] and developers are hoping that the current reservoir successfully treats nAMD for at least 24 mo. Since cells in the encapsulated chamber can be genetically modified to produce any molecule, the production of future DME drugs and even combination therapy may be possible. Should nAMD trials prove successful then testing for DME and DR will undoubtedly follow.
The ETDRS study focused on preventing vision loss so it enrolled patients with clinically significant macular edema (center-involving or center-threatening edema) and VA as good as 20/20[6]. Laser photocoagulation decreased the 3-year incidence of moderate vision loss (15 letters) by 50%, but is accompanied by the risk of laser-induced paracentral scotomas. In contrast, the anti-VEGF trials had upper VA limits of 20/32 or 20/40. Therefore, we have no good data from the anti-VEGF era regarding the best treatment for eyes with DME and VA better than 20/32.
Most physicians agree that laser photocoagulation is an excellent treatment for center-threatening DME with good VA. Laser produces a durable effect with few complications, but the best approach to center-involving DME and VA better than 20/32 is not known. Some physicians will treat symptomatic patients with center involving DME and excellent VA with anti-VEGF drugs despite the off-label indication. The DRCR.net Protocol V is comparing laser photocoagulation with intravitreal aflibercept for this population[36]. The study’s primary goal is to evaluate the effect of therapy on VA and DME, but the chance of decreasing the DR severity score makes this a very interesting study.
The DRS showed that timely pan-retinal photocoagulation of high-risk PDR decreases the risk of severe vision loss by over 50%[37]. Laser photocoagulation obliterates mid-peripheral areas of ischemic retina, thereby downregulating VEGF synthesis and promoting regression of neovascularization (NV). Unfortunately, broad areas of photocoagulation lead to permanently decreased peripheral vision and impaired night vision.
Anti-VEGF drugs involute optic disk neovascularization[38,39], but the effect of a single intravitreal injection is transient as retinal NV recurs by 12 wk[40]. Pre-operative and intra-operative injections of bevacizumab facilitate fibrovascular membrane dissection, and reduce intra-operative and post-operative bleeding. Anti-VEGFs have been used to prevent additional bleeding from PDR while waiting for vitreous hemorrhage to clear[41] though the long-term visual benefits with this approach are questionable.
The DRCR.net performed a multi-center (55 sites), randomized clinical trial comparing panretinal photocoagulation with intravitreal 0.5 mg ranibizumab in 305 patients with PDR[5]. PRP was performed at baseline and ranibizumab was given at baseline and q4week PRN. Eyes with DME in both groups were eligible to receive ranibizumab. The primary outcome was change in best corrected visual acuity (BCVA) and the secondary outcomes included area under the VA curve, peripheral visual field loss (as measured on Humphrey automated visual field testing), incidence of vitrectomy, development of DME, and persistent or new neovascularization. Improvements in BCVA for the ranibizumab and PRP groups were +2.2 and +0.2 letters respectively (95%CI: -0.5 to +5.0). The group receiving ranibizumab experienced less peripheral visual field sensitivity loss (-23 dB vs -422 dB; 95%CI: 213-531 dB; P < 0.001), fewer vitrectomies (4% vs 15%; 95%CI: 4%-15%; P < 0.001), and a lower incidence of DME (9% vs 28%). Ranibizumab treated eyes required a median of 7 injections through year 1 and 10 injections through year 2. Forty-five percent of eyes in the PRP group required additional laser and 53% of eyes required ranibizumab for DME. The authors concluded that ranibizumab may be a reasonable alternative to PRP through 2 years. The decreasing number of injections in year 2 suggests that some disease modulation occurs after 1 year of ranibizumab therapy.
These data are likely to promote a paradigm shift in the treatment of PDR. Compared to PRP, ranibizumab involutes NV while significantly preserving visual function. Ranibizumab may be a favorable alternative to PRP for compliant patients. Some physicians who prefer anti-VEGF therapy over laser will probably use bevacizumab and aflibercept, though data for these drugs are not yet available. Confirmatory studies with ranibizumab are needed as well as similarly structured trials with bevacizumab and aflibercept.
Anti-VEGF therapy has replaced macular laser photocoagulation for center-involving DME, but the anti-VEGF arms in all of the major anti-VEGF trials used laser as a rescue therapy. DRCR.net Protocol I combined ranibizumab with immediate or deferred (for at least 6 mo) laser[20]; Protocol T allowed laser after 6 mo[21]; RISE and RIDE allowed laser after 3 mo[3]; and VIVID/VISTA allowed laser after 3 mo[4].
RESTORE was the only trial to test 1 year of anti-VEGF monotherapy and these eyes experienced better visual improvement than the ranibizumab + laser arm[18]. Eyes in the ranibizumab + deferred laser arm of Protocol I achieved better visual improvement than those in the ranibizumab + prompt laser arm[20]. These data suggest that macular laser photocoagulation may actually limit visual acuity in eyes with DME. Some investigators perform early laser photocoagulation to decrease the number of necessary anti-VEGF injections, but Protocol I showed that patients in the prompt laser arm required 3 laser treatments in order to reduce the number of ranibizumab injections by 3[29]. Macular laser, therefore, appears to add little durability to a ranibizumab regimen.
A post hoc subset analysis of the RESTORE data showed that laser photocoagulation improved VA as well as monthly ranibizumab in eyes with central retinal thickness (CRT) < 400 μm[42]. This led the National Institute for Health Care Excellence (United Kingdom) to approve ranibizumab only for eyes with CRT > 400 μm. Comparable findings from RISE/RIDE and VIVID/VISTA have not been reported so the RESTORE data are not generalizable. Additional analyses of treatment effects according to baseline parameters such as CRT, ellipsoid zone integrity, and inner retinal edema need to be done.
Some physicians use the micropulse laser to treat macular edema due to DR and branch retinal vein occlusions. The laser delivers low-energy, millisecond bursts of laser to the photoreceptors and retinal pigment epithelium without causing permanent damage. Unlike standard photocoagulation, treatment to the same areas can be repeated. Small, retrospective studies have shown improvements in macular edema and visual acuity[43,44], but large, randomized, controlled trials against standard laser photocoagulation and in combination with anti-VEGF therapy need to be done.
Fluorescein angiographic studies with ultra-wide field (200°) cameras reveal large areas of peripheral retinal capillary non-perfusion in eyes with DME. DME recurs frequently in these eyes despite frequent anti-VEGF injections. Many investigators believe that these areas synthesize a continuous stream of VEGF that perpetuates the macular edema. Obliteration of these areas with scatter laser photocoagulation has produced inconsistent improvements in DME[45]. Randomized, controlled studies of image-guided peripheral laser photocoagulation are needed to assess the feasibility of this strategy.
Anti-VEGF therapy incompletely resolves edema in 20% to 40% of eyes and in the pivotal trials, laser photocoagulation after 3 to 6 mo was the only rescue therapy available for incomplete responders. Since macular laser photocoagulation has not been shown to improve VA, the best approach to incomplete responders is not known.
Now that ranibizumab and aflibercept have been Food and Drug Administration (FDA) approved for the treatment of DME, switching from a lower to a higher VEGF binding affinity drug (VEGF binding affinities: Bevacizumab < ranibizumab < aflibercept) has been studied[46]. This approach appears to improve macular thickening and modestly improve VA, but randomized, controlled studies need to be performed.
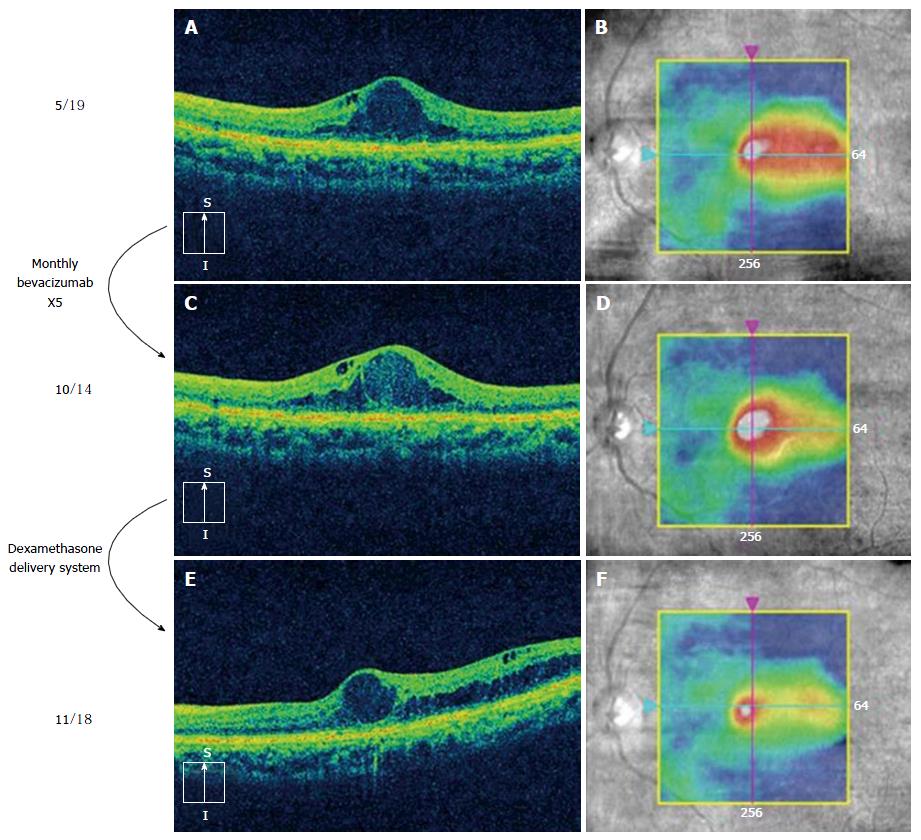
A popular approach to the treatment of incomplete responders is to switch from an anti-VEGF to a corticosteroid (usually DDS) (Figure 2) or add a corticosteroid (combination therapy). This frequently improves macular thickness, but additional VA gains are variable[47]. The fluocinolone acetonide (FA) insert works best in eyes with chronic DME, presumably because long-standing edema (> 3 years) is primarily driven by chemokines and not VEGF. However, the FA insert has not been studied in eyes that respond incompletely to anti-VEGF therapy so its efficacy in incomplete responders is unknown.
Pars plana vitrectomy has been performed in eyes with DME for over 20 years and it appears to be more commonly used in Europe and Japan than in the United States. Vitrectomy was originally studied in eyes with biomicroscopically visible vitreomacular traction[48], but its use has been expanded to eyes both with and without optical coherence tomography (OCT) identified traction. Many studies have reported excellent results[49,50], but a meta-analysis that included only 11 studies showed no significant response to surgery[51]. A DRCR.net study of eyes that had failed previous therapy showed that vitrectomy improves macular edema, but not mean VA[52]. The high variability in VA responses indicated that eyes did either very well or very poorly and the study was not able to predict responses based on baseline findings. Recent studies with pre-operative spectral domain OCT analyses of outer retina integrity (external limiting membrane and ellipsoid zone) suggest that vitrectomy holds promise as an early treatment for DME, but this remains to be proven[53].
We are fortunate to have a robust development pipeline for new DME drugs. Some of these drugs have already been mentioned in this manuscript, and others are listed in the Table 1. Most of these drugs are in phase I and II testing so clinical availability would not be expected for 5 to 10 years. Their potential uses vary from disease modulation in patients with early DR, to monotherapy or combination therapy for patients with established DME. Many of these drugs will not receive FDA approval, but others will provide us with better treatment options for patients with DR.
| Drug | Study design | Results |
| Danazol | 23 eyes: 12-wk placebo-control | Significant decreases in CRT (-86% vs -29%) and macular volume (P = 0.05) |
| Improvement in BCVA by 1 category (14% vs 47%) | ||
| Minocylcine | 5 eyes: Minocycline 100 mg BID for 6 mo | Improved BCVA and CRT compared to historic |
| controls | ||
| Loteprednol etabonate (topical) | 20 eyes: Single masked, 2-dose, randomized trial | Phase II clinical trial (KPI-121-C-004) underway |
| Dexamethasone phosphate | Iontophoresis driven into the eye EGP-437 | Positive results in 15 patients |
| PAN-90806 | 4 monotherapy arms in phase I/II trial | Maintenance therapy after single anti-VEGF injections |
| Diclofenac | 57 eyes: Intravitreal diclofenac vs bevacizumab | Diclofenac achieved better improvement in BCVA compared to bevacizumab (Δ -0.08 LogMAR vsΔ +0.04 LogMAR, P = 0.033) |
| Bevacizumab improved macular edema slightly | ||
| better | ||
| Sirolimus | Phase I trial: Dose- escalating, subconjunctival of intravitreal injections | Subconjunctival: median increase in BCVA was +4.0 letters at 45 d |
| Intravitreal: Median increase in BCVA was +4.0 letters at 90 d | ||
| Fasudil | Single intravitreal injections of fasudil with bevacizumab | At 4 wk ΔBCVA (0.84 ± 0.35 LogMAR to 0.49 ± 0.29 LogMAR; P = 0.003) and mean ΔCRT (448 ± 123 μm to 347 ± 76 μm; P = 0.001) |
| Luminate (ALG-001) | Phase IIb DME trial (targets integrin receptors) | Data expected third quarter of 2016 |
| Plasma kallekrein inhibitor (KVD001) | Phase II trials: Monotherapy for resistant DME combined with anti-VEGF | Phase I trial demonstrated safety after intravitreal injections |
| REGN910 (Ang 2 Ab) | Phase I trial | Completed. Now planning phase II trials for nAMD and DME |
With the introduction of potent pharmacotherapy we have witnessed dramatic improvements in the treatment of DR over the past decade. As we better understand the capabilities of available drugs and integrate them with treatments such as laser and surgery, and add new pharmacologic drugs to our treatment paradigms when they receive FDA approval, the future treatment for DR appears increasingly promising.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Parvizi N, Raghow RS, Tamemoto H S- Editor: Gong XM L- Editor: A E- Editor: Li D
| 1. | Sepúlveda J, Murray C. The state of global health in 2014. Science. 2014;345:1275-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Fong DS, Aiello LP, Ferris FL, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27:2540-2553. [PubMed] [Cited in This Article: ] |
| 3. | Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1019] [Cited by in F6Publishing: 1175] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 4. | Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247-2254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 573] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 5. | Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA. 2015;314:2137-2146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 492] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 6. | Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796-1806. [PubMed] [Cited in This Article: ] |
| 7. | Screening for retinopathy in the pediatric patient with type 1 diabetes mellitus. American Academy of Pediatrics. Sections on Endocrinology and Ophthalmology. Pediatrics. 1998;101:313-314. [PubMed] [Cited in This Article: ] |
| 8. | American Academy of Ophthalmology Retina Panel. Preferred Practice Pattern Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology, 2008. . [Cited in This Article: ] |
| 9. | Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, Klein R; American Diabetes Association. Diabetic retinopathy. Diabetes Care. 2003;26 Suppl 1:S99-S102. [PubMed] [Cited in This Article: ] |
| 10. | Canadian Ophthalmological Society Diabetic Retinopathy Clinical Practice Guideline Expert Committee, Hooper P, Boucher MC, Cruess A, Dawson KG, Delpero W, Greve M, Kozousek V, Lam WC, Maberley DA. Canadian Ophthalmological Society Evidence-based Clinical Practice Guidelines for the Management of Diabetic Retinopathy - executive summary. Can J Ophthalmol. 2012;47:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Mukamel DB, Bresnick GH, Wang Q, Dickey CF. Barriers to compliance with screening guidelines for diabetic retinopathy. Ophthalmic Epidemiol. 1999;6:61-72. [PubMed] [Cited in This Article: ] |
| 12. | Thompson AC, Thompson MO, Young DL, Lin RC, Sanislo SR, Moshfeghi DM, Singh K. Barriers to Follow-Up and Strategies to Improve Adherence to Appointments for Care of Chronic Eye Diseases. Invest Ophthalmol Vis Sci. 2015;56:4324-4331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Hipwell AE, Sturt J, Lindenmeyer A, Stratton I, Gadsby R, O’Hare P, Scanlon PH. Attitudes, access and anguish: a qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open. 2014;4:e005498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Palmer JJ, Chinanayi F, Gilbert A, Pillay D, Fox S, Jaggernath J, Naidoo K, Graham R, Patel D, Blanchet K. Trends and implications for achieving VISION 2020 human resources for eye health targets in 16 countries of sub-Saharan Africa by the year 2020. Hum Resour Health. 2014;12:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Rajalakshmi R, Arulmalar S, Usha M, Prathiba V, Kareemuddin KS, Anjana RM, Mohan V. Validation of Smartphone Based Retinal Photography for Diabetic Retinopathy Screening. PLoS One. 2015;10:e0138285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Walton OB, Garoon RB, Weng CY, Gross J, Young AK, Camero KA, Jin H, Carvounis PE, Coffee RE, Chu YI. Evaluation of Automated Teleretinal Screening Program for Diabetic Retinopathy. JAMA Ophthalmol. 2016;134:204-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Jiayi Wu, Jingmin Xin, Lai Hong, You J, Nanning Zheng. New hierarchical approach for microaneurysms detection with matched filter and machine learning. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:4322-4325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, Gerstner O, Bouazza AS, Shen H, Osborne A. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121:1045-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Boyer DS, Nguyen QD, Brown DM, Basu K, Ehrlich JS; RIDE and RISE Research Group. Outcomes with As-Needed Ranibizumab after Initial Monthly Therapy: Long-Term Outcomes of the Phase III RIDE and RISE Trials. Ophthalmology. 2015;122:2504-13.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL, Friedman SM, Glassman AR, Scott IU, Stockdale CR, Sun JK. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 403] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 21. | Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 668] [Cited by in F6Publishing: 652] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 22. | CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897-1908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1648] [Cited by in F6Publishing: 1929] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 23. | Stone TW, editor . America Society of Retina Specialists 2015 Preferences and Trends Membership Survey. Chicago, IL: American Society of Retina Specialists 2015; . [Cited in This Article: ] |
| 24. | Prünte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, Studnička J, Bezlyak V, Parikh S, Stubbings WJ, Wenzel A. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100:787-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 25. | Heier JS, Bressler NM, Avery RL, Bakri SJ, Boyer DS, Brown DM, Dugel PU, Freund KB, Glassman AR, Kim JE. Comparison of Aflibercept, Bevacizumab, and Ranibizumab for Treatment of Diabetic Macular Edema: Extrapolation of Data to Clinical Practice. JAMA Ophthalmol. 2016;134:95-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 26. | Boyer DS, Yoon YH, Belfort R, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 546] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 27. | Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, Garretson B, Gupta A, Hariprasad SM, Bailey C. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125-2132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 28. | Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG, Weber M, Danis RP, Kuppermann BD, Bailey C. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology. 2014;121:1892-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, Ferris FL, Glassman AR, Maturi RK, Melia M. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119:2312-2318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 30. | Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122:803-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Campochiaro PA, Channa R, Berger BB, Heier JS, Brown DM, Fiedler U, Hepp J, Stumpp MT. Treatment of diabetic macular edema with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am J Ophthalmol. 2013;155:697-704, 704.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Allergan. Safety and Efficacy of Abicipar Pegol in Patients With Neovascular Age-related Macular Degeneration. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2016 Jan 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT02462486 NLM Identifier: NCT02462486. [Cited in This Article: ] |
| 33. | Helzner J. Promising Data on Sustained-release Lucentis. [accessed 2016 Jan 24]. Available from: http://www.retinalphysician.com/articleviewer.aspx?articleID=111136. [Cited in This Article: ] |
| 34. | ClinicalTrials ; Neurotech Pharmaceuticals. Study of the Intravitreal Implantation of NT-503-3 Encapsulated Cell Technology (ECT) for the Treatment of Recurrent Choroidal Neovascularization (CNV) Secondary to Age-related Macular Degeneration (AMD). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2016 Jan 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT02228304 NLM Identifier: NCT02228304. [Cited in This Article: ] |
| 35. | Kauper K, McGovern C, Sherman S, Heatherton P, Rapoza R, Stabila P, Dean B, Lee A, Borges S, Bouchard B. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2012;53:7484-7491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Diabetic Retinopathy Clinical Research Network. Treatment for CI-DME in Eyes With Very Good VA Study (Protocol V). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2016 Jan 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT01909791 NLM Identifier: NCT01909791. [Cited in This Article: ] |
| 37. | Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88:583-600. [PubMed] [Cited in This Article: ] |
| 38. | Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352-354. [PubMed] [Cited in This Article: ] |
| 39. | Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275-278. [PubMed] [Cited in This Article: ] |
| 40. | Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina. 2006;26:1006-1013. [PubMed] [Cited in This Article: ] |
| 41. | Zhang ZH, Liu HY, Hernandez-Da Mota SE, Romano MR, Falavarjani KG, Ahmadieh H, Xu X, Liu K. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol. 2013;156:106-115.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 969] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 43. | Nicolò M, Musetti D, Traverso CE. Yellow micropulse laser in diabetic macular edema: a short-term pilot study. Eur J Ophthalmol. 2014;24:885-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Kwon YH, Lee DK, Kwon OW. The short-term efficacy of subthreshold Micropulse yellow (577-nm) laser photocoagulation for diabetic macular edema. Korean J Ophthalmol. 2014;28:379-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Suñer IJ, Peden MC, Hammer ME, Grizzard WS, Traynom J, Cousins SW. RaScaL: A Pilot Study to Assess the Efficacy, Durability, and Safety of a Single Intervention with Ranibizumab plus Peripheral Laser for Diabetic Macular Edema Associated with Peripheral Nonperfusion on Ultrawide-Field Fluorescein Angiography. Ophthalmologica. 2014; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 46. | Lim LS, Ng WY, Mathur R, Wong D, Wong EY, Yeo I, Cheung CM, Lee SY, Wong TY, Papakostas TD. Conversion to aflibercept for diabetic macular edema unresponsive to ranibizumab or bevacizumab. Clin Ophthalmol. 2015;9:1715-1718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Maturi RK, Bleau L, Saunders J, Mubasher M, Stewart MW. A 12-month, single-masked, randomized controlled study of eyes with persistent diabetic macular edema after multiple anti-VEGF injections to assess the efficacy of the dexamethasone-delayed delivery system as an adjunct to bevacizumab compared with continued bevacizumab monotherapy. Retina. 2015;35:1604-1614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 48. | Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753-759. [PubMed] [Cited in This Article: ] |
| 49. | Landers MB III, Kon Graversen VA, Stewart MW. Early vitrec-tomy for DME: does it have a role? Sometimes vitrectomy can be first-line treatment. Part 1 of 2. Retinal Physician, 2013: 46-53. . [Cited in This Article: ] |
| 50. | Landers MB III, Kon Graversen VA, Stewart MW. Early vitrec-tomy for DME: does it have a role? Sometimes vitrectomy can be first-line treatment. Part 2 of 2. Retinal Physician, 2013: 56-60. . [Cited in This Article: ] |
| 51. | Simunovic MP, Hunyor AP, Ho IV. Vitrectomy for diabetic macular edema: a systematic review and meta-analysis. Can J Ophthalmol. 2014;49:188-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Flaxel CJ, Edwards AR, Aiello LP, Arrigg PG, Beck RW, Bressler NM, Bressler SB, Ferris FL, Gupta SK, Haller JA. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina. 2010;30:1488-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 53. | Chhablani JK, Kim JS, Cheng L, Kozak I, Freeman W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250:1415-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |