Copyright
©The Author(s) 2015.
World J Diabetes. Jun 25, 2015; 6(6): 807-827
Published online Jun 25, 2015. doi: 10.4239/wjd.v6.i6.807
Published online Jun 25, 2015. doi: 10.4239/wjd.v6.i6.807
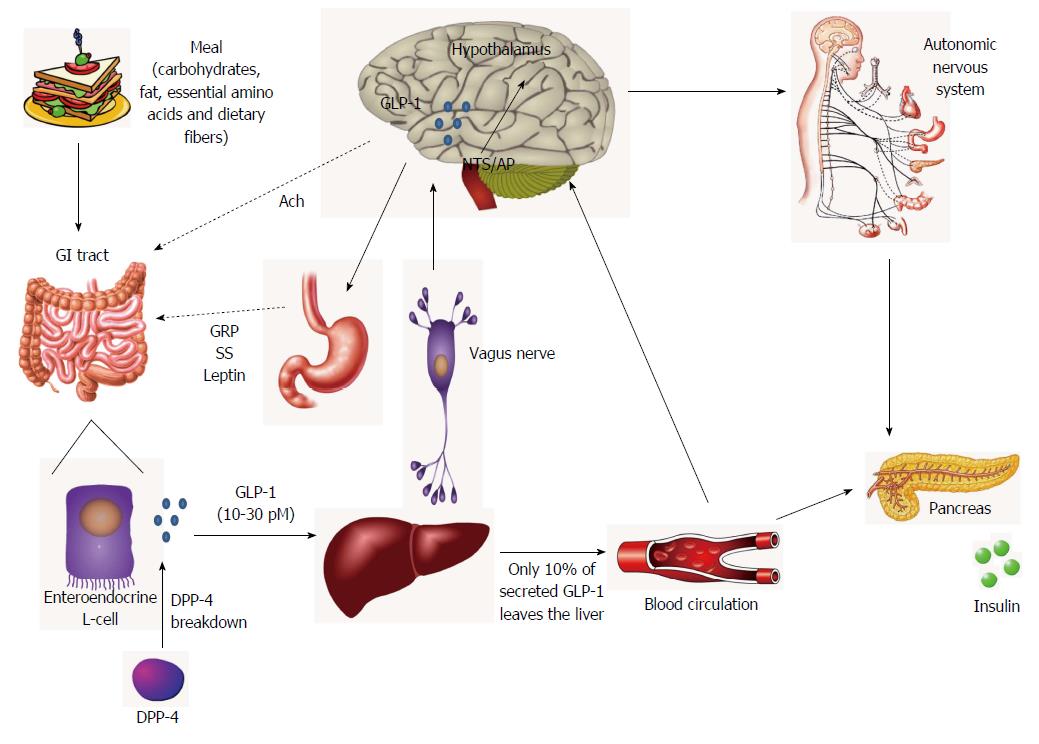
Figure 1 The gut-brain axis for the actions of glucagon-like peptide-1.
After a meal ingestion, gastrointestinal (GI) tract is rapidly stimulated and glucagon-like peptide-1 (GLP-1) is secreted in the gut lumen by enteroendocrine L-cells. Besides the direct interaction of nutrients with L-cells, neural (acetylcholine) and endocrine (gastrin-releasing peptide, somatostatin and leptin) mechanisms are also involved in the control of GLP-1 secretion after food intake. Bioactive GLP-1 diffuses into the capillaries, immediately beginning to be degraded by dipeptidyl peptidase-4, so that more than 50% of the hormone is inactivated before reaching the portal circulation. In the liver, a further large amount is truncated, thus only 10% of the secreted GLP-1 leaves the liver and enters the systemic circulation and may reach the pancreas, the brain and other tissues via the endocrine pathway. However, the passage of GLP-1 through the hepatoportal vein activates vagal afferents nerves that initiate a neural signal towards the brain. In the central nervous system, the metabolic information is received by the solitary tract nucleus and the AP in the brainstem, which synthesize and project the GLP-1 to the hypothalamus. The GLP-1 receptor signaling is involved in the central control of energy homeostasis and food intake, and several autonomous functions, such as glucose-dependent stimulation of insulin secretion and inhibition of glucagon secretion in the pancreas, cardiovascular effects, regulation of gastric emptying and of endogenous glucose production in liver and glucose uptake and storage in muscle and adipose tissue. GRP: Gastrin-releasing peptide; Ach: Acetylcholine; SS: Somatostatin; DPP-4: Dipeptidyl peptidase-4; AP: Area postrema.
- Citation: Candeias EM, Sebastião IC, Cardoso SM, Correia SC, Carvalho CI, Plácido AI, Santos MS, Oliveira CR, Moreira PI, Duarte AI. Gut-brain connection: The neuroprotective effects of the anti-diabetic drug liraglutide. World J Diabetes 2015; 6(6): 807-827
- URL: https://www.wjgnet.com/1948-9358/full/v6/i6/807.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i6.807