Copyright
©2013 Baishideng Publishing Group Co.
World J Diabetes. Dec 15, 2013; 4(6): 310-318
Published online Dec 15, 2013. doi: 10.4239/wjd.v4.i6.310
Published online Dec 15, 2013. doi: 10.4239/wjd.v4.i6.310
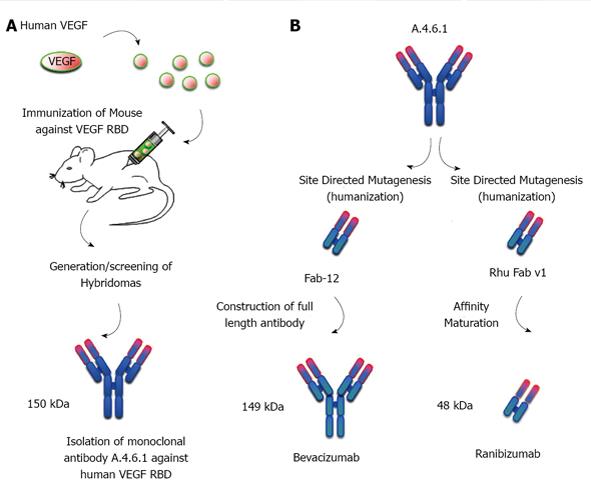
Figure 2 Development of humanized neutralizing monoclonal antibodies against vascular endothelial growth factor.
A: A polypeptide from the vascular endothelial growth factor (VEGF) receptor-binding domain (RBD) was used to immunize mice. The spleen from the mice was isolated and lymphocytes were cultured with immortal myeloma cell lines. Fusion of the cell lines results in the generation of hybridomas. Supernatants from the hybridoma cultures are tested for antibodies that bind specifically and with high affinity to VEGF. This resulted in the identification of the parental mouse monoclonal antibody, A.4.6.1; B: Site directed mutagenesis was then used to “humanize” the Fab (epitope-binding) fragment of A.4.6.1. Fab-12 was used to construct the full-length antibody, bevacizumab (left). Rhu Fab v1 was further affinity matured to produce the Fab fragment, ranibizumab (right).
- Citation: Krispel C, Rodrigues M, Xin X, Sodhi A. Ranibizumab in diabetic macular edema. World J Diabetes 2013; 4(6): 310-318
- URL: https://www.wjgnet.com/1948-9358/full/v4/i6/310.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i6.310