Copyright
©2011 Baishideng Publishing Group Co.
World J Diabetes. Nov 15, 2011; 2(11): 176-188
Published online Nov 15, 2011. doi: 10.4239/wjd.v2.i11.176
Published online Nov 15, 2011. doi: 10.4239/wjd.v2.i11.176
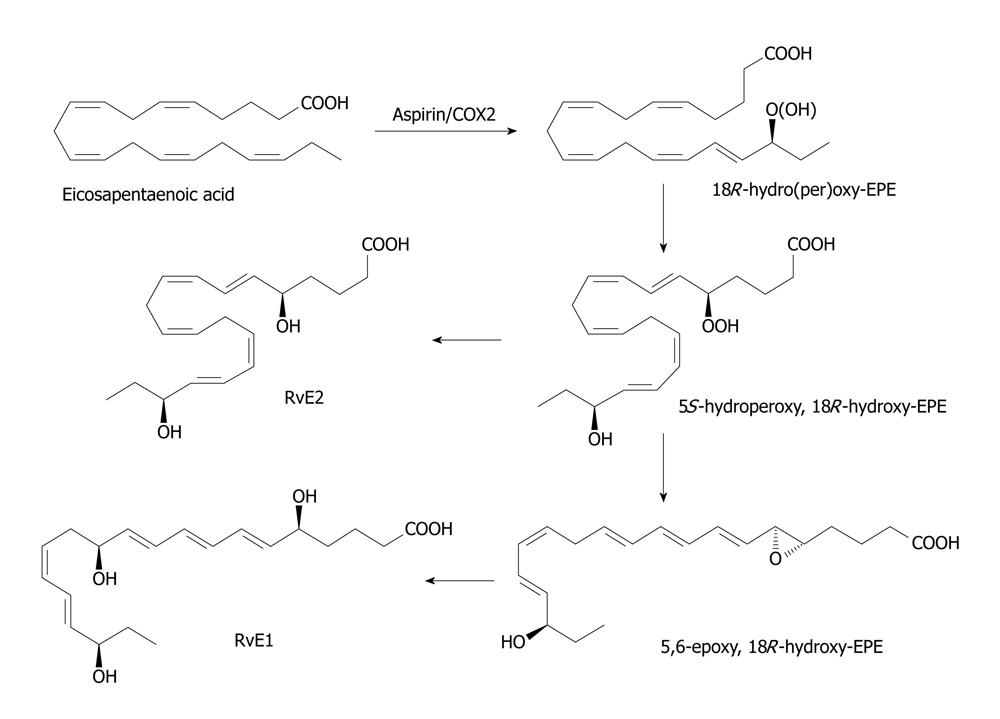
Figure 3 Scheme showing the formation of resolvin E derived from eicosapentaenoic acid.
In the endothelial cells, the cyclo-oxygenase (COX)-2 enzyme that has been acetylated introduces an 18R hydroperoxy-group into the eicosapentaenoic acid molecule (c.f. the role of aspirin in the biosynthesis of the epi-lipoxins). This is reduced to the corresponding hydroxy compound before a 5S-hydroperoxy group is introduced into the molecule by the action of 5-lipoxygenase as in the biosynthesis of leukotrienes. A further reduction step produces 15S,18R-dihydroxy-EPE or resolvin E2. Alternatively, the 5S-hydrpperoxy, 18R-hydroxy-EPE intermediates is converted to a 5,6-epoxy fatty acids in polymorphonuclear leukocytes I humans and eventually to 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eiocsapentaenoic acid or resolvin E1 by process similar to the formation of leukotrienes in leukocytes.
- Citation: Das UN. A defect in the activities of Δ6 and Δ5 desaturases and pro-resolution bioactive lipids in the pathobiology of non-alcoholic fatty liver disease. World J Diabetes 2011; 2(11): 176-188
- URL: https://www.wjgnet.com/1948-9358/full/v2/i11/176.htm
- DOI: https://dx.doi.org/10.4239/wjd.v2.i11.176