Copyright
©The Author(s) 2025.
World J Diabetes. May 15, 2025; 16(5): 99473
Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.99473
Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.99473
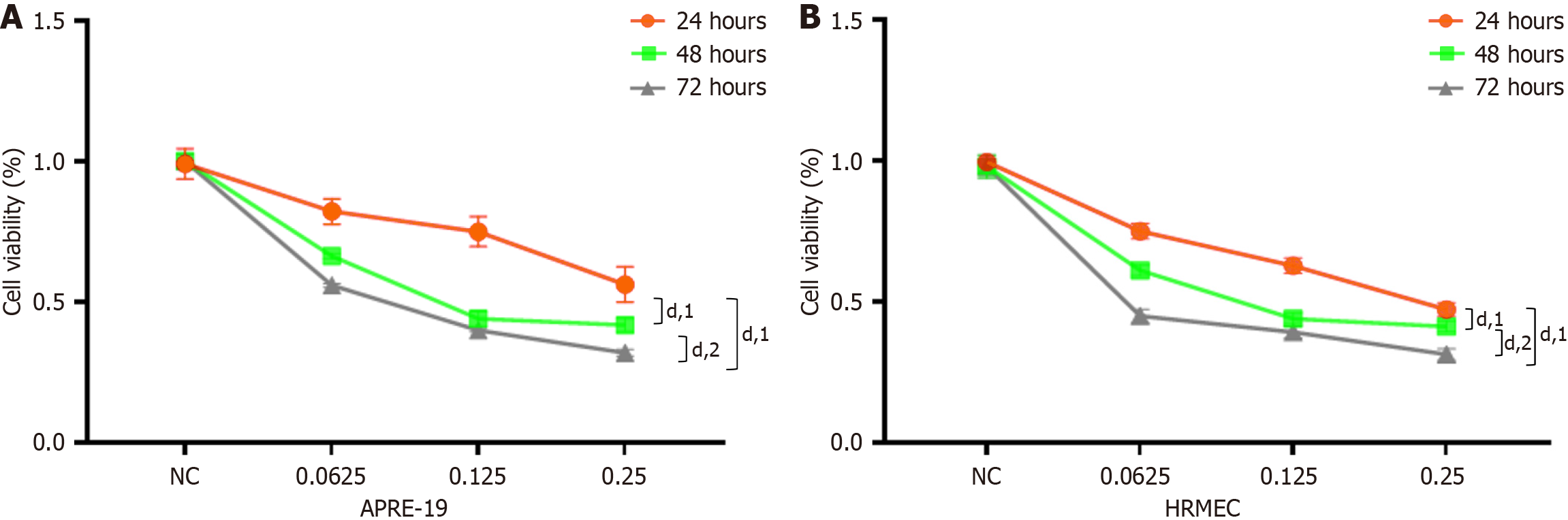
Figure 1 Effect of ranibizumab on the viability of adult retinal pigment epithelial 19 cells and human retinal microvascular endothelial cells.
Cell viability was assessed using the Cell Counting Kit-8 assay (n = 3, independent experiments). A: Effect of ranibizumab (0 mg/mL, 0.0625 mg/mL, 0.125 mg/mL, or 025 mg/mL) treatment on adult retinal pigment epithelial 19 (ARPE-19) cell viability; B: Effect of ranibizumab (0 mg/mL, 0.0625 mg/mL, 0.125 mg/mL, or 025 mg/mL) treatment on human retinal microvascular endothelial cell (HRMEC) viability. All results are expressed as the mean ± SD. dP < 0.0001. 1P vs 24 hour group. 2P vs 48 hour group. NC: Untreated group.
- Citation: Lin YT, Tan J, Tao YL, Hu WW, Wang YC, Huang J, Zhou Q, Xiao A. Effect of ranibizumab on diabetic retinopathy via the vascular endothelial growth factor/STAT3/glial fibrillary acidic protein pathway. World J Diabetes 2025; 16(5): 99473
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/99473.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.99473