Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1259
Peer-review started: March 28, 2023
First decision: May 8, 2023
Revised: May 25, 2023
Accepted: June 19, 2023
Article in press: June 19, 2023
Published online: August 15, 2023
Processing time: 136 Days and 1.4 Hours
Globally, patients with diabetes suffer from increased disease severity and mortality due to coronavirus disease 2019 (COVID-19). Old age, high body mass index (BMI), comorbidities, and complications of diabetes are recognized as major risk factors for infection severity and mortality.
To investigate the risk and predictors of higher severity and mortality among in-hospital patients with COVID-19 and type 2 diabetes (T2D) during the first wave of the pandemic in Dubai (March–September 2020).
In this cross-sectional nested case-control study, a total of 1083 patients with COVID-19 were recruited. This study included 890 men and 193 women. Of these, 427 had T2D and 656 were non-diabetic. The clinical, radiographic, and laboratory data of the patients with and without T2D were compared. Independent predictors of mortality in COVID-19 non-survivors were identified in patients with and without T2D.
T2D patients with COVID-19 were older and had higher BMI than those without T2D. They had higher rates of comorbidities such as hypertension, ischemic heart disease, heart failure, and more life-threatening complications. All laboratory parameters of disease severity were significantly higher than in those without T2D. Therefore, these patients had a longer hospital stay and a significantly higher mortality rate. They died from COVID-19 at a rate three times higher than patients without. Most laboratory and radiographic severity indices in non-survivors were high in patients with and without T2D. In the univariate analysis of the predictors of mortality among all COVID-19 non-survivors, significant associations were identified with old age, increased white blood cell count, lym-phopenia, and elevated serum troponin levels. In multivariate analysis, only lymphopenia was identified as an independent predictor of mortality among T2D non-survivors.
Patients with COVID-19 and T2D were older with higher BMI, more comorbidities, higher disease severity indices, more severe proinflammatory state with cardiac involvement, and died from COVID-19 at three times the rate of patients without T2D. The identified mortality predictors will help healthcare workers prioritize the management of patients with COVID-19.
Core Tip: Globally, patients with diabetes suffer from increased disease severity and mortality due to coronavirus disease 2019 (COVID-19). Old age, high body mass index (BMI), comorbidities, and complications of diabetes were recognized as major risk factors for infection severity and mortality. To identify the independent predictors of mortality in patients with type 2 diabetes (T2D) in Dubai, United Arab Emirates, we performed a cross-sectional nested case-control study during the first wave of the pandemic. It seems that the mortality of patients with T2D is driven by a significantly higher pro-inflammatory response to COVID-19 as evidenced by higher C-reactive protein, white blood cell, and lymphopenia. Mortality also seems to be synergistic with the comorbidities and complications of T2D in patients with COVID-19. The identified mortality predictors will help healthcare workers prioritize the management of patients with COVID-19.
- Citation: Alawadi F, Bashier A, Bin Hussain AA, Al-Hashmi N, Bachet FAT, Hassanein MMA, Zidan MA, Soued R, Khamis AH, Mukhopadhyay D, Abdul F, Osama A, Sulaiman F, Farooqi MH, Bayoumi RAL. Risk and predictors of severity and mortality in patients with type 2 diabetes and COVID-19 in Dubai. World J Diabetes 2023; 14(8): 1259-1270
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1259.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1259
It has been established that older age, higher body mass index (BMI), and comorbidities such as diabetes mellitus, hypertension, cardiovascular disease (CVD), and chronic kidney disease (CKD) are the major risk factors for severity and mortality of coronavirus disease 2019 (COVID-19) infection in patients in China[1,2], United Kingdom[3,4], United States[5-7], Sweden[8] and many other countries[9,10]. Evidence of higher severity and mortality of COVID-19 in patients with diabetes has been reported in many of the aforementioned studies and confirmed by others[11-14]. Systematic reviews[15] and meta-analyses[16-20] have established that the severity of COVID-19 in patients with diabetes is observed across all ethnicities. Similar observations were recorded in the United Arab Emirates during the first and subsequent waves of the pandemic[21-26].
The aim of this retrospective study was to determine the risk and predictors that drove the higher severity among in-hospital patients with COVID-19 and type 2 diabetes (T2D) and to describe the relationship between diabetes and all-cause mortality during the first wave of the pandemic in Dubai, United Arab Emirates.
During the early phase of the COVID-19 pandemic, Dubai Hospital, where the study was conducted, was among the three main centers designated for the isolation and admission of all patients with COVID-19 in the Emirate of Dubai. Dubai Hospital is a specialized hospital equipped with 625 beds and incorporates 26 surgical and medical departments, including the intensive care unit (ICU), cardiology, oncology, nephrology, and endocrinology. During the first wave of the COVID-19 pandemic, most hospital wards were converted into isolation units. In this cross-sectional nested case-control study, 1083 patients with COVID-19 admitted to Dubai Hospital between March 21st and September 30th, 2020, were recruited.
All patients positive for COVID-19 were admitted to hospital. Patients were tested using reverse transcription-polymerase chain reaction (RT-PCR) using a nasopharyngeal swab for respiratory tract infection to test for COVID-19. A total of 1083 patients were randomly recruited for the study. This study included 890 men and 193 women. Of these patients, 427 had T2D and 656 did not.
Patients with type 1 diabetes were excluded. This was based on their previous medical history collected from the SALAMA Electronic Health Records (EHR) and confirmed upon admission. The following clinical data were obtained from the EHR: Age (13–87 years), sex (men/women), nationality (emirates/expatriates), and comorbidities [hypertension, ischemic stroke, cardiomyopathy, heart failure, chronic obstructive pulmonary disease, asthma, and diabetic ketoacidosis]. The following presenting symptoms were recorded at admission: Fever, cough, shortness of breath, sore throat, chest pain, myalgia, headache, chills, fatigue, malaise, loss of appetite, runny nose, abdominal pain, loss of taste, loss of smell, vomiting, diarrhea, dizziness, confusion, skin rash, and arthralgia. Noninvasive ventilation, intubation, and admission to the ICU were also recorded.
The mean ages of the patients were 51 and 39 years for those with and without T2D, respectively. Demographic features and the presence of co-morbidities, such as a prior diagnosis of cardiac disease, hypertension, diabetes, or respiratory disease, and any medication used were recorded. Patients were further categorized as asymptomatic, mild, moderate, severe, or critical according to the National Institutes of Health categorization of COVID-19 severity[26], as follows:
Asymptomatic: Patients have no symptoms but have tested positive for COVID-19.
Mild: Patients have symptoms and signs such as fever, cough, sore throat, malaise, headache, or muscle pain, but no shortness of breath, dyspnea, or abnormal chest radiography.
Moderate: Patients had clinical or imaging findings suggestive of lower respiratory disease but maintained an oxygen saturation of > 93% on room air.
Severe: The respiratory rate (RR) of the patients was above 30/min, oxygen saturation was ≤ 93%, PaO2/FiO2 was < 300, and/or pneumonic infiltrates involving > 50% of the lungs were observed.
Critical: Patients suffer respiratory failure, septic shock, and/or multiple organ dysfunction.
The vital signs recorded at the time of admission included blood pressure, heart rate, RR, SpO2, and temperature.
Data were collected from all patients with and without T2D from the Dubai Hospital SALAMA Electronic Medical Record System. The information collected was categorized into demographic data, medical and glycemic histories, physical examination results, comorbidities, laboratory investigation results, chest radiographs, complications, and treatment protocols. The collected data were entered into an SPSS data collection sheet explicitly designed for this study. All patient data were de-identified throughout the collection, categorization, and creation of the database. The reporting of this study conformed to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines[27].
Laboratory measurements recorded for patients with COVID-19 included complete blood count, ferritin, absolute lymphocyte count, lactate dehydrogenase, procalcitonin, C-reactive protein (CRP), creatine phosphokinase, D-dimer, pro-BNP, serum creatinine, fasting blood glucose, and HbA1C.
Chest X-ray scoring: A chest radiograph severity scoring system was used to determine the severity of the pneumonia. The images were also classified as exhibiting ground-glass opacities, reticular patterns, or consolidations and as normal, mild, moderate, or severe.
The management and Treatment of COVID-19 followed the United Arab Emirates Guidelines[28]. which are based on the National Institutes of Health (NIH) guidelines (NIH, 2021)[29]. After a thorough assessment of clinical severity, all patients with COVID-19 were treated (March–September 2020) with a standard combination of hydroxychloroquine sulfate and Kaletra antiviral (lopinavir/ritonavir). Therefore, there were no differences in drug management between patients with and without T2D. Patients were considered cured after two consecutive negative (determined through RT-PCR) nasopharyngeal swabs following clinical recovery.
Data were analyzed using SPSS for Windows version 28.0 (SPSS Inc., Chicago, IL, United States). Categorical variables are described as percentages. Continuous variables were described using a measure of tendency and dispersion. Continuous data were tested for normality using the Kolmogorov-Smirnov test. The Mann-Whitney U test and t-test were used when appropriate to compare the means between continuous variables. Categorical variables were cross-tabulated to examine the independence between variables, the χ2 test or Fisher’s exact test was used as appropriate.
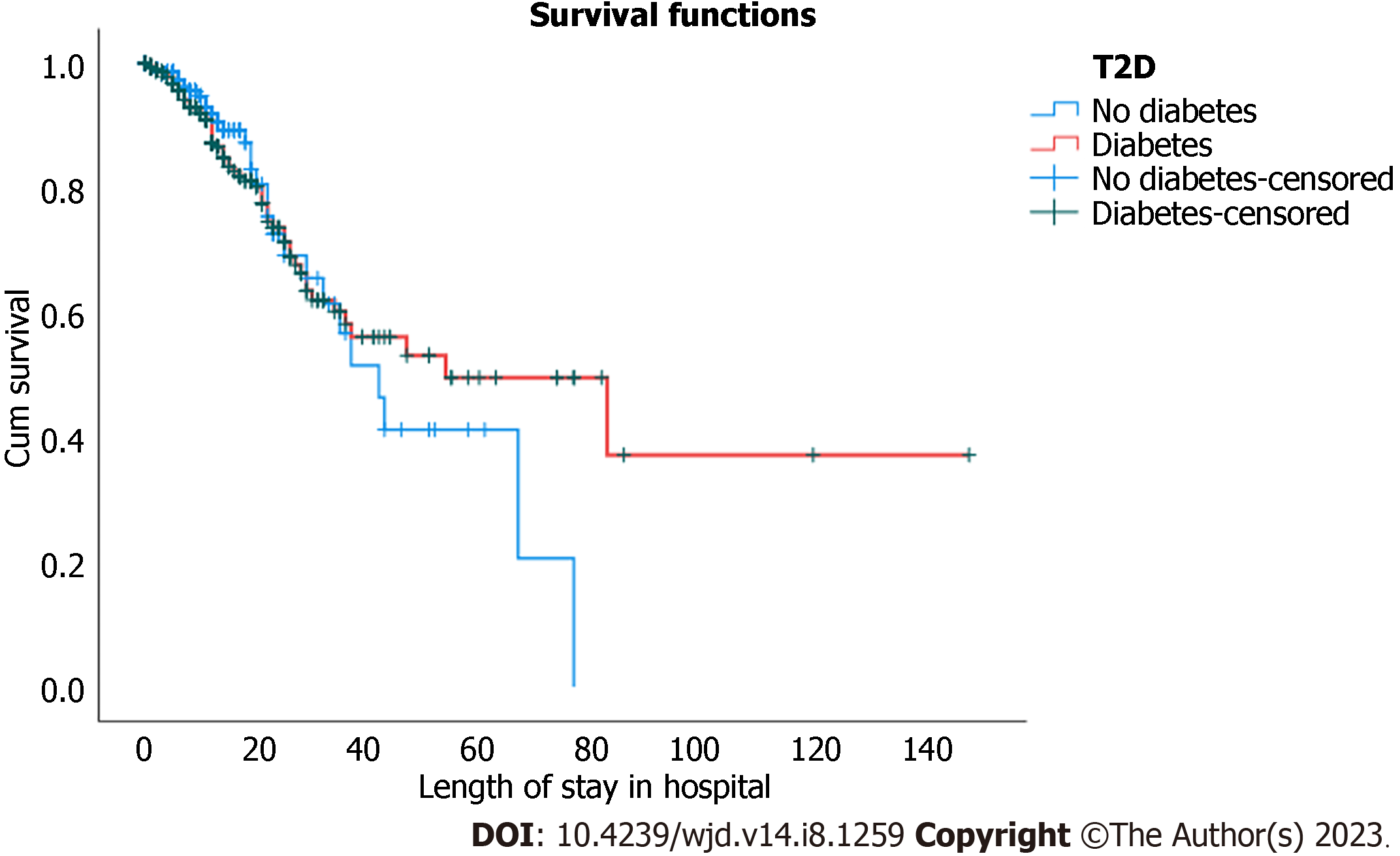
Kaplan-Meier estimates were used to examine the effect of T2D on survival. The estimate was based on the serial time to death of patients with T2D and controls. The results of the computed model are visualized in a survival plot (Figure 1). In addition to the Kaplan-Meier analysis, univariate and multivariate analyses containing all the variables that differed statistically between survivors and non-survivors were performed to identify the independent predictors of mortality among patients with COVID-19 and T2D. Statistical significance was set at P < 0.05.
A total of 1083 patients (890 men and 193 women) diagnosed with COVID-19 were enrolled in this study. Among the men, 378 (42%) had T2D, whereas among the women, only 49 (25%) had T2D. The mean age of patients with T2D is 51.4 (± 11.2), whereas for patients without T2D is 39.4 (± 13.5) (P < 0.001). There was also a significant difference in BMI between patients with T2D [28.80 (± 5.47)] compared to patients without T2D [27.20 (± 4.94)] (P < 0.001).
The demographic characteristics and comorbidities of patients with T2D and patients without are shown in Table 1. Patients with T2D and COVID-19 had higher rates of comorbidities, such as hypertension, ischemic heart disease, and heart failure compared to patients with COVID-19 without T2D.
| Characteristics | T2D (n = 427) | Without T2D (n = 656) | P valuea |
| A: Demographics and comorbidities [n (%)] | |||
| Gender | No (%) | ||
| Male | 378 (42.5) | 512 (57.5) | |
| Female | 49 (25.4) | 144 (74.6) | < 0.001 |
| Nationality | |||
| United Arab Emirates | 23 (59) | 16 (41) | |
| Expatriate | 404 (38.7) | 640 (61.3) | 0.009 |
| Hypertension | 165/427 (38.6) | 88/656 (13.4) | < 0.001 |
| Ischemic heart disease | 21/427 (4.9) | 3/656 (0.5) | < 0.001 |
| Cardiomyopathy | 2/427 (0.5) | 0/656 | 0.155 |
| Heart failure | 13/427 (3) | 4/656 (0.6) | 0.002 |
| COPD | 2/427 (0.5) | 1/656 (0.2) | 0.344 |
| Asthma | 10/427 (2.3) | 18/656 (2.7) | 0.421 |
| B: Symptoms and signs [n (%)] | |||
| Fever | 358 (85.9) | 528 (82.8) | 0.105 |
| Cough | 303 (72.7) | 388 (60.8) | < 0.001 |
| Sore throat | 52 (12.5) | 150 (23.5) | < 0.001 |
| Running nose/rhinorrhoea | 17 (4.1) | 40 (6.3) | 0.079 |
| Shortness of breath (dyspnoea) | 276 (66.2) | 250 (39.2) | < 0.001 |
| Chest pain | 22 (5.3) | 29 (4.5) | 0.344 |
| Chills | 13 (3.1) | 35 (5.5) | 0.047 |
| Headache | 24 (5.8) | 74 (11.6) | < 0.001 |
| Fatigue | 92 (22.1) | 116 (18.2) | 0.071 |
| Malaise | 8 (1.9) | 15 (2.4) | 0.405 |
| Nausea | 11 (2.6) | 17 (2.6) | 0.572 |
| Loss of appetite/anorexia | 19 (4.6) | 22 (3.4) | 0.226 |
| Loss of taste | 1 (0.2) | 6 (0.9) | 0.164 |
| Anosmia/loss of smell | 1 (0.2) | 6 (0.9) | 0.164 |
| Abdominal pain | 12 (2.9) | 46 (7.2) | 0.001 |
| Vomiting | 39 (9.4) | 47 (7.4) | 0.150 |
| Diarrhoea | 26 (6.2) | 34 (5.3) | 0.312 |
| Myalgia | 123 (29.5) | 180 (28.2) | 0.351 |
| Arthralgia/joint pain | 1 (0.2) | 2 (0.2) | 0.635 |
| Dizziness | 10 (2.4) | 21 (3.3) | 0.259 |
| Confusion | 4 (1.0) | 2 (0.3) | 0.216 |
| Skin rash | 1 (0.2) | 1 (0.2) | 0.635 |
| C: Laboratory measurements [mean (± SD)] | |||
| Haemoglobin (g/dL) | 12.42 (2.07) | 13.45 (1.84) | < 0.001 |
| MCV (fL) | 85.66 (37.79) | 84.54 (7.74) | 0.01 |
| WBC (109/L) | 9.62 (7.87) | 8.31 (7.25) | < 0.001 |
| Lymphocyte (%) | 14.57 (8.52) | 18.46 (10.08) | < 0.001 |
| Absolute lymphocyte count | 1.05 (0.82) | 1.26 (0.78) | < 0.001 |
| Random blood glucose (mg/dL) | 222.53 (114.59) | 119.16 (42.75) | < 0.001 |
| Fasting blood glucose POCT (mg/dL) | 192.06 (88.88) | 126.77 (48.38) | < 0.001 |
| HbA1C (%) | 9.26 (2.44) | 5.74 (0.47) | < 0.001 |
| CRP (mg/L) | 86.38 (88.05) | 50.23 (64.68) | < 0.001 |
| Troponin (ng/mL) | 180.10 (917.58) | 18.29 (64.11) | < 0.001 |
| D-Dimer (mcg/mL) | 4.14 (6.43) | 2.46 (5.04) | < 0.001 |
| Pro-calcitonin (ng/mL) | 0.97 (4.2) | 0.61 (3.18) | < 0.001 |
| Pro-BNP (pg/mL) | 1948.56 (4602.46) | 1533.62 (6449.57) | 0.008 |
| Ferritin (mcg/L) | 1526.7 (2153.81) | 1231.68 (3268.65) | < 0.001 |
| LDH (U/L) | 444.93 (371.05) | 355.55 (243.98) | < 0.001 |
| LDH-Peak (U/L) | 429.27 (393.39) | 366.62 (585.83) | < 0.001 |
| CPK peak (U/L) | 597.8 (1090.39) | 505.5 (2055.57) | 0.022 |
| Creatinine (mg/dL) | 1.87 (2.12) | 1.16 (1.44) | < 0.001 |
| D: Chest radiographs, ventilation and disease outcome [No (%)] | |||
| Normal chest radiograph | 16 (3.8) | 99 (16.5) | < 0.001 |
| Mild consolidation [1-2 zones] | 174 (41.7) | 342 (57.1) | |
| Moderate consolidation [2-3 zones] | 183 (43.9) | 125 (20.9) | |
| Severe consolidation [3-4 zones] | 44 (10.6) | 33 (5.5) | |
| E: SpO2 on admission | |||
| ≤ 94 | 84 (19.7) | 64 (9.8) | < 0.001 |
| > 94 | 343 (80.3) | 592 (90.2) | |
| F: Ventilation and/or intubation | |||
| O2 mask | 58 (13.6) | 43 (6.6) | < 0.001 |
| Nasal canula | 311 (72.8) | 361 (55) | < 0.001 |
| Intubation | 42 (9.8) | 15 (2.3) | < 0.001 |
| G: Complications & Outcomes | |||
| Diabetic keto acidosis | 7 (4.5) | 0 | 0.01 |
| Chronic kidney failure | 37 (10.5) | 16 (3) | < 0.001 |
| Length of hospital stay, mean (days) | 14.90 (17.31) | 7.49 (10.22) | < 0.001 |
| Death: No (%) | 63/427 (14.8) | 32/656 (4.9) | < 0.001 |
Only dyspnea, cough, sore throat, and headache were significantly more frequent in patients with T2D (Table 1).
All 18 Laboratory measurements indicative of disease severity were significantly higher among patients with T2D (Table 1).
Chest radiographs of patients with COVID-19 and T2D exhibited significantly more lung consolidation, ground-glass appearance, and severity than patients without (Table 1). Consequently, COVID-19 patients with T2D had significantly lower SpO2 saturation and required more noninvasive assisted ventilation as well as frequent mechanical ventilation. They also had more life-threatening complications, such as diabetic ketoacidosis and acute kidney failure. Therefore, these patients had longer hospital stays and significantly higher mortality rates.
All patients with COVID-19 were treated (March-September 2020) with a standard combination of hydroxychloroquine sulfate and Kaletra antiviral medication (lopinavir/ritonavir). Therefore, there were no differences in drug management between patients with and without T2D.
In general, patients with COVID-19 with T2D were 12 years older than those without T2D (Table 1). In addition, non-survivors were older than survivors in both groups (Table 2). On admission, there were no significant differences in the symptoms between the two groups.
| Characteristics | T2D (n = 427) | Without T2D (n = 656) | ||||
| Survivors (n = 364) | Non-survivors (n = 63) | P value | Survivors (n = 624) | Non-survivors (n = 32) | P valuea < 0.001 | |
| A: Demographics and comorbidities [n (%)] | ||||||
| Males: No (%) | 325 (86) | 53 (14) | 0.164 | 484 (94.1) | 30 (5.9) | 0.072 |
| Females: No (%) | 39 (79.6) | 10 (20.4) | 140 (98.6) | 2 (1.4) | ||
| Age: Yr (± SD) | 50.76 (10.92) | 54.86 (12.58) | 0.02 | 38.83 (13.42) | 48.58 (12.43) | < 0.001 |
| BMI (±SD) | 28.81 (5.53) | 28.69 (5.08) | 0.841 | 27.15 (4.95) | 28.03 (5.05) | 0.400 |
| Hypertension, No (%) | 137 (37.6) | 28 (44.4) | 0.188 | 77 (12.4) | 11 (30.6) | 0.005 |
| B: Symptoms and signs [n (%)] | ||||||
| Fever | 306 (86.4) | 52 (82.5) | 0.26 | 502 (82.8) | 26 (81.0) | 0.484 |
| Cough | 256 (72.3) | 47 (74.6) | 0.418 | 359 (59.2) | 29 (90.6) | < 0.001 |
| Sore throat | 50 (14.1) | 2 (3.2) | 0.007 | 146 (24.1) | 4 (12.5) | 0.093 |
| Dyspnea | 224 (63.3) | 52 (82.5) | 0.002 | 222 (36.6) | 28 (87.5) | < 0.001 |
| Headache | 23 (6.5) | 1 (1.6) | 0.097 | 73 (12) | 1 (3.1) | 0.095 |
| C: Laboratory measurements [mean (± SD)] | ||||||
| Hemoglobin (g/dL) | 12.69 (2.06) | 10.95 (1.36) | < 0.001 | 13.6 (1.7) | 10.7 (1.2) | < 0.001 |
| MCV (fL) | 85.98 (41) | 83.95 (7.28) | 0.815 | 84.4 (7.6) | 86.2 (10) | 0.223 |
| WBC (109/L) | 9.02 (6.56) | 12.86 (12.41) | < 0.001 | 7.6 (4.75) | 18.9 (20.4) | < 0.001 |
| Lymphocytes (%) | 15.48 (8.59) | 9.55 (6.1) | < 0.001 | 18.79 (9.7) | 13.1 (13.3) | < 0.001 |
| Absolute lymphocyte | 1.13 (0.84) | 0.57 (0.5) | < 0.001 | 1.3 (0.7) | 1.2 (1.6) | < 0.001 |
| Random glucose (mg/dL) | 221.52 (115.69) | 227.94 (109.3) | 0.442 | 118.8 (43.6) | 124.4 (62.3) | 0.026 |
| Fasting glucose (mg/dL) | 107.46 (114.85) | 118.1 (121.74) | 0.191 | 118.8 (43.6) | 52.5 (68) | < 0.001 |
| HbA1C (%) | 9.32 (2.4) | 8.87 (2.64) | 0.13 | 5.73 (0.5) | 5.9 (0.4) | 0.586 |
| CRP (mg/L) | 76.23 (77.72) | 141.1 (116.77) | < 0.001 | 44.6 (56) | 138 (108) | < 0.001 |
| Troponin (ng/mL) | 167.19 (990.22) | 238.21 (470) | < 0.001 | 15.9 (64.5) | 37.6 (58.4) | < 0.001 |
| D-Dimer (mcg/mL) | 3.09 (4.53) | 9.56 (10.79) | < 0.001 | 1.78 (3.84) | 8.9 (9.1) | < 0.001 |
| Procalcitonin (ng/mL) | 0.83 (4.32) | 1.75 (3.45) | < 0.001 | 0.51 (3.01) | 1.9 (4.72) | < 0.001 |
| Pro-BNP (pg/mL) | 1225.01 (3572) | 3862.9 (6248.31) | 0.007 | 1324.5 (7323) | 2109.1 (2985.8) | < 0.001 |
| Ferritin (mcg/L) | 1277.9 (1599.2) | 2888 (3749) | < 0.001 | 856.2 (1179) | 5549.9 (10016) | < 0.001 |
| LDH (U/L) | 416.41 (365.96) | 611.04 (360.04) | < 0.001 | 321.1 (164.7) | 785 (521.5) | < 0.001 |
| LDH-Peak (U/L) | 402.02 (394.6) | 605.2 (339.82) | < 0.001 | 346.3 (587.3) | 644.1 (491.3) | < 0.001 |
| CPK peak (U/L) | 464.9 (970.7) | 1163.5 (1369.5) | < 0.001 | 432.8 (2145.6) | 1041.2 (1085) | < 0.001 |
| Creatinine (mg/dL) | 1.61 (2.01) | 3.22 (2.17) | < 0.001 | 1.02 (1.22) | 3.2 (2.55) | < 0.001 |
| D: Chest radiographs, ventilation and disease outcome [n (mean ± SD)] | ||||||
| E: Chest radiograph | ||||||
| Normal X-ray | 15 (4.2) | 1 (1.6) | 0.021 | 99 (17.4) | 0 | < 0.001 |
| Mild | 156 (43.9) | 18 (29) | 335 (59) | 7 (22.6) | ||
| Moderate | 152 (42.8) | 31 (50) | 11 (19.5) | 14 (45.2) | ||
| Severe | 32 (9) | 12 (19.4) | 23 (4) | 10 (32.3) | ||
| F: SpO2 on admission | ||||||
| ≤ 94 | 56 (15.4) | 28 (44.4) | 574 (92.6) | 18 (50) | < 0.001 | |
| > 94 | 308 (84.6) | 35 (55.6) | < 0.001 | 46 (7.4) | 18 (50) | |
| G: Ventilation and/or intubation | ||||||
| O2 mask | 32 (8.8) | 26 (41.3) | < 0.001 | 23 (3.7) | 20 (55.6) | < 0.001 |
| Nasal canula | 269 (73.9) | 42 (66.7) | 0.15 | 340 (54.8) | 21 (58.3) | 0.408 |
| Intubation | 21 (5.8) | 21 (33.3) | < 0.001 | 6 (1.0) | 9 (25) | < 0.001 |
| H: Complications & outcomes | ||||||
| Diabetic ketoacidosis | 3 (2.3) | 4 (14.3) | 0.02 | |||
| Chronic kidney failure | 19 (6.4) | 18 (31) | < 0.001 | 11 (2.2) | 5 (15.2) | 0.002 |
| Length of hospital stay: Mean (SD) | 14.47 (17.9) | 17.02 (14) | 0.005 | 6.47 (8.53) | 32 (20.06) | < 0.001 |
Most laboratory indicators of disease severity were equally high among the COVID-19 non-survivors in each group (Table 2). The association of radiographic and severity indices of chest infection with mortality in patients with COVID-19 was also equally high among the patients in each group. Therefore, most risk factors for severity and predictors of mortality in T2D non-survivors and controls were similar, with few exceptions (Table 2).
The relationship between random blood glucose, fasting blood glucose and blood HbA1C, and mortality among patients with COVID-19 and T2D was tested using the Mann-Whitney U test and found to be statistically insignificant.
Predictors of mortality were determined in non-survivors of COVID-19 with and without T2D, using univariate analysis. Among the eight clinical and 12 Laboratory risk factors (Table 2), significant associations of mortality of COVID-19 in patients with and without T2D were identified with advanced age, increased total white blood cell (WBC) count, lymphopenia, and elevated serum troponin. In multivariate analysis, only lymphopenia was identified as a predictor of mortality in patients with T2D.
Kaplan-Meier analysis was used to compare survival against serial time to death between patients with and without T2D (Figure 1). The median time of death among patients without T2D is less than that among patients with T2D [42 d with 95%CI: 32.2–51.8 and 54 d with 95%CI (23.3–84.7)]; respectively. This difference was not statistically significant because 95% of the CIs overlapped. However, the number of deaths was significantly higher among patients with T2D [63 (14.8%)] than among patients without T2D [36 (5.5%)], with P < 0.001 (Table 3).
| Total number of patients (n = 1083) | Patients with T2D (n = 427) | Patients without T2D (n = 656) | Ratio: T2D:Non-T2D | |
| 0-5 d | 15 | 10 | 5 | 3.1 |
| 6-10 d | 18 | 12 | 6 | 3.1 |
| 11-15 d | 20 | 15 | 5 | 4.6 |
| 16-20 d | 8 | 4 | 4 | 1.53 |
| 21-25 d | 13 | 9 | 4 | 3.5 |
| 26-30 d | 8 | 7 | 1 | 10.8 |
| > 30 d | 13 | 6 | 7 | 1.3 |
| Total | 95 | 63 | 32 | 3.1 |
The overall ratio of COVID-19 patients who died among patients with T2D was three times that of patients without T2D (Table 3). The ratio remained high in each successive 5-d interval for the first 4 wk of admission but decreased thereafter.
In this cross-sectional nested case-control study, we determined the risk factors and predictors that drove higher disease severity and mortality among in-hospital patients with COVID-19 and T2D during the first wave of the pandemic in Dubai, United Arab Emirates. Patients with T2D and COVID-19 seem to have higher rates of comorbidities such as hypertension, ischemic heart disease, and heart failure compared to patients with COVID-19 without T2D. Older men with T2D and high BMI were more prone to severe COVID-19. Dyspnea, cough, sore throat, and headache were significantly more frequent in patients with T2D. All laboratory measurements indicative of disease severity were significantly higher in patients with T2D. On chest radiographs, patients with COVID-19 infection with T2D showed significantly more lung consolidation, glassy appearance, and severity than patients without T2D. Consequently, patients with COVID-19 with T2D had significantly lower SpO2 saturation and required more noninvasive assisted ventilation as well as frequent mechanical ventilation. They also had more life-threatening complications, such as diabetic ketoacidosis and acute kidney failure. Therefore, these patients had more ICU admissions, longer hospital stays, and significantly higher mortality rates.
Most laboratory indicators and radiographic and severity indices of chest infection in non-survivors were equally high in patients with COVID-19 with and without T2D. Therefore, we used Kaplan-Meier analysis to explain survival against serial time to death between patients with and without T2D. Although the median time of death among patients without T2D is lower than that among patients with T2D (42 vs 54 d), the number of deaths was significantly higher among patients with T2D [63/427 (14.8%)] than among patients without T2D [32/656 (4.9%)]. The overall ratio of patients with T2D dying is three times higher than that of patients without T2D. The ratio remained high in each successive 5-d interval for the first 4 wk of admission, but, decreased thereafter.
In addition to Kaplan-Meier analysis of the effect of T2D on survival, univariate and multivariate analyses were performed, including all the variables that differed statistically between survivors and non-survivors, to identify independent predictors of mortality among patients with COVID-19. Initially, predictors of mortality were examined using univariate analysis. Among eight clinical and 12 Laboratory risk factors, significant associations of mortality of COVID-19 in patients with and without T2D were identified with advancing age, increased total WBC count, lym-phopenia, and elevated serum troponin. In multivariate analysis, only lymphopenia was identified as a predictor of mortality in patients with T2D.
In this study, we found no relationship between glycemic control and mortality. It cannot be implied that the poorer the glycemic control, the higher the mortality rate. Hyperglycemia per se is not an independent predictor of mortality. In contrast, it seems that the mortality of patients with T2D is driven by a much higher inflammatory response to COVID-19 as evidenced by higher CRP levels, higher WBC counts, and lymphopenia. It is also possible that mortality is associated with cardiac damage as evidenced by elevated serum troponin and synergized by the large number of comorbidities and complications observed in patients with T2D[30].
Similar investigations conducted overseas[1-20] and in the United Arab Emirates[21-26], have reported that the prevalence of diabetes in patients with COVID-19 does not differ from that in the general population, indicating that the primary risk of COVID-19 infection is not increased in patients with diabetes. It seems that the increased risk of COVID-19 severity and mortality in patients with diabetes is due to older age and comorbidities, such as hypertension, CVD, CKD, and obesity, in addition to a severe proinflammatory state and cardiac involvement. The risk that patients with diabetes face is that they are more likely to have worse complications and not have a greater chance of contracting the virus[31].
This study demonstrated that patients with COVID-19 with T2D had more comorbidities, higher disease severity, and died at three times the rate of patients without T2D. Identifying the predictors of mortality may allow healthcare workers to prioritize vaccination and implement early management strategies for patients with COVID-19 patients and T2D to offset this calamity.
The authors acknowledge the several limitations of this study. It is possible that there was a selection bias in this study, as it was a short-term cross-sectional nested case-control study in which data were collected over a short period in a pandemic environment. In this cross-sectional study, it was not possible to establish causal inferences or analyze temporal changes. Patients could not be followed-up with after discharge, limiting the possibility of further outcomes. Despite these limitations, this study provided data on a large cohort of patients with COVID-19 and T2D with results similar to those of many internationally acknowledged studies.
Patients with COVID-19 and T2D were older with higher BMI, more comorbidities, higher disease severity indices, more severe proinflammatory state with cardiac involvement, and died from COVID-19 at three times the rate of patients without T2D. The identified mortality predictors will help healthcare workers prioritize the management of patients with COVID-19.
Patients with diabetes suffer higher morbidity and mortality from corona virus disease 2019 (COVID-19). Despite better outcomes due to vaccination, many patients with diabetes continue to suffer from the disease. It is imperative therefore, to continue investigating the risk and predictors of COVID-19 severity in this vulnerable group, to help shaping better management of the disease.
Identifying independent predictors of mortality from COVID-19 will allow healthcare workers to prioritize vaccination, implement early management strategies for patients with COVID-19 and type 2 diabetes (T2D), and offset disease severity and mortality.
To identify independent mortality predictors among in-hospital patients with COVID-19 and T2D during the first wave of the pandemic (March–September 2020) in Dubai, United Arab Emirates.
In this cross-sectional nested case-control study, a total of 1083 patients with COVID-19 were recruited. Of these, 427 had T2D and 656 were non-diabetic. The clinical, radiographic, and laboratory data of the patients with and without T2D were compared. Independent predictors of mortality in COVID-19 non-survivors were identified in patients with and without T2D.
Patients with T2D and COVID-19 were older and had a higher body mass index than patients without T2D. They had higher rates of comorbidities such as hypertension, ischemic heart disease, heart failure, and more life-threatening complications. All laboratory parameters of disease severity were significantly higher than in those without T2D. Therefore, these patients had a longer hospital stay and a significantly higher mortality rate. These patients died from COVID-19 at three times the rate of patients without T2D. In the univariate analysis of the independent predictors of mortality among all COVID-19 non-survivors, significant associations were identified with old age, increased white blood cell count, lymphopenia, and elevated serum troponin levels. In multivariate analysis, only lymphopenia was identified as an independent predictor of mortality among T2D non-survivors.
It seems that the increased severity and mortality of patients with COVID-19 and T2D is due to older age and comorbidities such as hypertension, cardiovascular disease, chronic kidney disease, and obesity, with an added severe proinflammatory state.
It is necessary to further investigate the factors that heighten the pro-inflammatory state, driving higher mortality among patients with T2D and COVID-19.
We are grateful to administrative and nursing staff of Dubai Hospital at Dubai Academic Health Corporation, for supporting the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aizawa T, Japan; Cai L, United States; Naswhan AJ, Qatar; Tzamaloukas AH, United States S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18173] [Article Influence: 3634.6] [Reference Citation Analysis (0)] |
| 2. | Shi Q, Zhang X, Jiang F, Hu N, Bimu C, Feng J, Yan S, Guan Y, Xu D, He G, Chen C, Xiong X, Liu L, Li H, Tao J, Peng Z, Wang W. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes Care. 2020;43:1382-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 3. | Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, Wareham NJ, Young B, Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 663] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 4. | Dennis JM, Mateen BA, Sonabend R, Thomas NJ, Patel KA, Hattersley AT, Denaxas S, McGovern AP, Vollmer SJ. Type 2 Diabetes and COVID-19-Related Mortality in the Critical Care Setting: A National Cohort Study in England, March-July 2020. Diabetes Care. 2021;44:50-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 5. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6500] [Article Influence: 1300.0] [Reference Citation Analysis (0)] |
| 6. | Kim D, Alshuwaykh O, Sandhu KK, Dennis BB, Cholankeril G, Ahmed A. Trends in All-Cause and Cause-Specific Mortality Among Individuals With Diabetes Before and During the COVID-19 Pandemic in the U.S. Diabetes Care. 2022;45:e107-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Clouston SAP, Luft BJ, Sun E. Clinical risk factors for mortality in an analysis of 1375 patients admitted for COVID treatment. Sci Rep. 2021;11:23414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Rawshani A, Kjölhede EA, Rawshani A, Sattar N, Eeg-Olofsson K, Adiels M, Ludvigsson J, Lindh M, Gisslén M, Hagberg E, Lappas G, Eliasson B, Rosengren A. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: A nationwide retrospective cohort study. Lancet Reg Health Eur. 2021;4:100105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Nlandu Y, Mafuta D, Sakaji J, Brecknell M, Engole Y, Abatha J, Nkumu JR, Nkodila A, Mboliassa MF, Tuyinama O, Bena D, Mboloko Y, Kobo P, Boloko P, Tshangu J, Azika P, Kanku JP, Mafuta P, Atantama M, Mavungu JM, Kitenge R, Sehli A, Van Eckout K, Mukuku C, Bergeret L, Benchetritt D, Kalifa G, Rodolphe A, Bukabau J. Predictors of mortality in COVID-19 patients at Kinshasa Medical Center and a survival analysis: a retrospective cohort study. BMC Infect Dis. 2021;21:1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 10. | Heald AH, Jenkins DA, Williams R, Sperrin M, Mudaliar RN, Syed A, Naseem A, Bowden Davies KA, Peng Y, Peek N, Ollier W, Anderson SG, Delanerolle G, Gibson JM. Mortality in People with Type 2 Diabetes Following SARS-CoV-2 Infection: A Population Level Analysis of Potential Risk Factors. Diabetes Ther. 2022;13:1037-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 477] [Article Influence: 95.4] [Reference Citation Analysis (1)] |
| 12. | Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ, Sattar N, Young B, Valabhji J. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 609] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 13. | Kristan MM, Kim YK, Nelson T, Moxley MC, Yip TC, Munir K, Malek R. Predictors of Severe COVID-19 in Patients With Diabetes: A Multicenter Review. Endocr Pract. 2021;27:842-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Huang J, Zhu L, Bai X, Jia X, Lu Y, Deng A, Li J, Jin S. Multidimensional Analysis of Risk Factors for the Severity and Mortality of Patients with COVID-19 and Diabetes. Infect Dis Ther. 2020;9:981-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Singh BB, Ward MP, Lowerison M, Lewinson RT, Vallerand IA, Deardon R, Gill JPS, Singh B, Barkema HW. Meta-analysis and adjusted estimation of COVID-19 case fatality risk in India and its association with the underlying comorbidities. One Health. 2021;13:100283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Miller LE, Bhattacharyya R, Miller AL. Diabetes mellitus increases the risk of hospital mortality in patients with Covid-19: Systematic review with meta-analysis. Medicine (Baltimore). 2020;99:e22439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Kastora S, Patel M, Carter B, Delibegovic M, Myint PK. Impact of diabetes on COVID-19 mortality and hospital outcomes from a global perspective: An umbrella systematic review and meta-analysis. Endocrinol Diabetes Metab. 2022;5:e00338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Corona G, Pizzocaro A, Vena W, Rastrelli G, Semeraro F, Isidori AM, Pivonello R, Salonia A, Sforza A, Maggi M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev Endocr Metab Disord. 2021;22:275-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 19. | Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 512] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 20. | Gupta P, Gupta M, KAtoch N, Garg K, Garg B. A Systematic Review and Meta-analysis of Diabetes Associated Mortality in Patients with COVID-19. Int J Endocrinol Metab. 2021;19:e113220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Elemam NM, Hannawi H, Salmi IA, Naeem KB, Alokaily F, Hannawi S. Diabetes mellitus as a comorbidity in COVID-19 infection in the United Arab Emirates. Saudi Med J. 2021;42:170-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Hannawi S, Hannawi H, Naeem KB, Elemam NM, Hachim MY, Hachim IY, Darwish AS, Al Salmi I. Clinical and Laboratory Profile of Hospitalized Symptomatic COVID-19 Patients: Case Series Study From the First COVID-19 Center in the UAE. Front Cell Infect Microbiol. 2021;11:632965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Deeb A, Khawaja K, Sakrani N, AlAkhras A, Al Mesabi A, Trehan R, Kumar PC, Babiker Z, Nagelkerke N, Fru-Nsutebu E. Impact of Ethnicity and Underlying Comorbidity on COVID-19 Inhospital Mortality: An Observational Study in Abu Dhabi, UAE. Biomed Res Int. 2021;2021:6695707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Nair SC, Gasmelseed HI, Khan AA, Khafagy IN, Sreedharan J, Saleem AA, Abdrhman HI, Alhosani AH, Siddiqua AR, Ahmed AR, Shubbar AI, Aleissaee AR, Alanqar AW, Hamadeh AM, Safdani FA, Habbal FW, Choker HB, Bashir KM, Alblooshi MA, Farajallah MM, Alzaabi MN, Shil RS, Alshehhi SS, Douleh WF. Assessment of mortality from COVID-19 in a multicultural multi-ethnic patient population. BMC Infect Dis. 2021;21:1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Hosani FA, Aden B, Memari SA, Mazrouei SA, Ajab S, Abid M, Alsuwaidi AR, Grivna M, Paulo MS, Sheek-Hussein M. Epidemiology of asymptomatic and symptomatic Coronavirus Disease 2019 confirmed cases in the Emirate of Abu Dhabi, United Arab Emirates: Observational study. Medicine (Baltimore). 2021;100:e25219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Bhatti R, Khamis AH, Khatib S, Shiraz S, Matfin G. Clinical Characteristics and Outcomes of Patients With Diabetes Admitted for COVID-19 Treatment in Dubai: Single-Centre Cross-Sectional Study. JMIR Public Health Surveill. 2020;6:e22471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3438] [Cited by in RCA: 6080] [Article Influence: 337.8] [Reference Citation Analysis (0)] |
| 28. | UAE. National Guidelines for Clinical Management and Treatment of COVID-19. Mar 19, 2020. [cited 23 May 2023]. Available from: https://www.dhcc.ae/gallery/National%20Guidelines%20for%20Clinical%20Management%20and%20Treatment%20of%20COVID-19_English.pdf. [DOI] [Full Text] |
| 29. | National Institutes of Health. Clinical Spectrum, COVID-19 Treatment Guidelines. Mar 6, 2023. [cited 01 June 2023]. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. |
| 30. | Soliman AT, Prabhakaran Nair A, Al Masalamani MS, De Sanctis V, Abu Khattab MA, Alsaud AE, Sasi S, Ali EA, Ola A H, Iqbal FM, Nashwan AJ, Fahad J, El Madhoun I, Yassin MA. Prevalence, clinical manifestations, and biochemical data of type 2 diabetes mellitus versus nondiabetic symptomatic patients with COVID-19: A comparative study. Acta Biomed. 2020;91:e2020010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 31. | Diabetes UK. Exploring research: Can coronavirus cause diabetes, or make it worse? Dec 14, 2022. [cited 01 June 2023]. Available from: https://www.diabetes.org.uk/about_us/news/new-worse-cases-coronavirus. |