Published online Apr 15, 2023. doi: 10.4239/wjd.v14.i4.435
Peer-review started: November 8, 2022
First decision: November 27, 2022
Revised: December 11, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: April 15, 2023
Processing time: 154 Days and 1.1 Hours
Diabetic sensorimotor polyneuropathy is an important risk factor for foot ulceration and amputation. Thus, patients with diabetes should be screened for this disorder according to local guidelines. An obstacle to the diagnosis of this disease may be the lack of unified diagnostic criteria due to the lack of properly validated scales used for assessment.
To validate both sections (A and B) of the Michigan Neuropathy Screening Instrument (MNSI) in Polish (PL) patients with diabetes.
A cross-sectional study using a test (A1, B1) and re-test (A2, B2) formula was performed in 80 patients with diabetes. The gold standard used for neuropathy detection was a nerve conduction study (NCS) which was performed in all participants. Reliability of the MNSI-PL was assessed using the Cronbach’s alpha, Kuder-Richardson formula 20 (KR-20), split-half reliability, the Gottman split-half tests, and correlation between first and second half was accessed. Stability was assessed using an intraclass correlation coefficient (ICC). For external validation, we used simple linear correlation, binomial regression, and agreement between two different tools using a Bland-Altman plot analysis.
The scale was internally consistent (Cronbach’s alpha for the full scale: 0.81 for A and 0.87 for B). MNSI-PL scores in test/retest showed high stability (ICC = 0.73 for A and ICC = 0.97 for B). The statistically important correlations between MNSI-PL and NCS were found for B1, B2, and A1 (P < 0.005). The cut-off points of ≥ 3 for section A (sensitivity of 90%-100%; specificity of 33%-40%) and ≥ 2 for section B (sensitivity of 81%-84%; specificity of 60%-70%) were obtained during neuropathy detection.
The MNSI-PL is a reliable and valid instrument in screening for diabetic neuropathy.
Core Tip: The Michigan Neuropathy Screening Instrument (MNSI) is a collective tool for assessing the peripheral nervous system in patients with diabetes mellitus and is widely used to evaluate patients in many countries. In Poland, the MNSI has not yet been validated, thus is a problem when using it in daily practice and for research purposes. We concluded that the Polish MNSI is a reliable and accurate screening tool for peripheral neuropathy, and we hope that it can be used by our colleagues in their studies. We also proposed cutoff points of both scales for patients and for physicians, hoping that MNSI can be used by other authors from different countries
- Citation: Sutkowska E, Marciniak D, Koszewicz M, Dziadkowiak E, Budrewicz S, Biernat K, Kuciel N, Mazurek J, Hap K. Validity and reliability of the Polish version of the Michigan Neuropathy Screening Instrument. World J Diabetes 2023; 14(4): 435-446
- URL: https://www.wjgnet.com/1948-9358/full/v14/i4/435.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i4.435
A peripheral, symmetric sensorimotor polyneuropathy is the most common type of neuropathy in patients with diabetes mellitus (DM)[1]. Epidemiological data show that it occurs in approximately 28%-50% of patients with DM[2,3] depending on the population studied. It may begin developing in prediabetes[4], as carbohydrate disturbances start a few years before the diagnosis of type 2 DM (T2DM). According to guidelines, patients with T2DM should be screened for complications at the time of diagnosis. An examination in patients with type 1 DM (T1DM) should be performed approximately 5 years after the beginning of the disease. To minimize the risk of severe complications and to monitor neuropathy severity, regular inspections and examinations are recommended to be performed at least annually according to the International Working Group on the Diabetic Foot (2019)[5] as well as many local guidelines including those from the Polish Diabetes Association[6].
Peripheral neuropathy can be bothersome and significantly reduce a patient’s quality of life. It is difficult to effectively treat and up to 50% of diabetic peripheral neuropathies can be asymptomatic. As a result of the deformation and loss of protective sensation caused by peripheral neuropathy, ulceration and finally amputation can occur[7-9] , leading to the patient’s disability. Therefore, the early detection of this characteristic disorder of the peripheral nervous system is important using proven and readily available tools. The examiner should ask patients about their subjective feeling (symptoms) and perform tests to assess the function of small and large fibers[10]. All necessary tests are included in the Michigan Neuropathy Screening Instrument (MNSI)[11], which is a collective tool used to assess the peripheral nervous system in patients with DM and has been validated for patients in many countries. So far, this tool has not been validated to assess neuropathy in Poland. The use of MNSI may improve the diagnostic process of peripheral neuropathy and could be helpful in future studies. The reliability and precision of this tool was validated in previous studies[11-13]. The first section of the MNSI is subjective and consists of 15 self-administered questions, while the second section is a physical assessment that should be completed by health professionals.
According to the information from the dedicated website (https://eprovide.mapi-trust.org/instruments/michigan-neuropathy-screening-instrument), the MNSI has been translated into Chinese, Czech, Finnish, French, German, Hungarian, Portuguese, Romanian, Spanish, and Turkish languages and validated. After confirming that the scale has not been validated in Polish (PL), the investigators obtained consent to validate the MNSI. The original English version is freely available for download from the Michigan Diabetes Research Center’s (MDRC’s) website. The project was financially supported by the Ministry of Health Subvention according to the number SUBZ.C310.22.075 from the IT Simple System of the Wroclaw Medical University.
Our cross-sectional study was conducted at the University Rehabilitation Centre at Wroclaw Medical University in collaboration with the Department of Neurology. The study was approved by the Wroclaw Medical University’s ethics committee (approval no: 1007/2021). The patients were recruited from NZOZ Nowy Dwor in Wroclaw, Poland, and in accordance with the Declaration of Helsinki. All patients included in the study provided written informed consent to participate in the study.
Between January and April 2022, 89 patients met the inclusion criteria and were eligible for participation in the study. However, 8 of them did not come to the clinic within the stipulated time frame. Thus, a total of 81 patients signed the consent and participated in the study. One of the patients recalled during the course of the project that she had received chemotherapy for cancer treatment and was excluded from the analysis, this left a population of 80 participants.
For the patient’s section of the MNSI, the only change proposed by both the patients and the committee was to replace the wording “legs” with “legs and feet”. This is because for many people the word “legs” is commonly used to refer to the area from the groin to the ankles. For this reason, the question about feeling in the legs may not take into account problems with the feet. The same problem was reported by the team from Portugal[14]. Patients were also asked what exactly the authors meant in point number 13: “Are you able to sense your feet when you walk?” (original version). In PL, it is hard to translate this into a simple and short form. This was also identified in the retranslation because after retranslation the sentence was: “Do you feel the right sensation in your feet when walking?” and in the committee’s opinion, it may be misleading. Thus, we suggested that even though this part of the scale is self-administered, the practitioner could help patients to understand the question. For the examiner’s section, we stressed that each of the abnormalities should be recorded if present. This was not clear in the translated version according to the diabetologist.
In the instruction “How to Use the Michigan Neuropathy Screening Instrument”, the following elements were changed after translation into PL to better reflect what was done: (1) In the part entitled “vibration sensation”: We stressed in PL the word “bilaterally”; and (2) In the part entitled “ankle reflexes”: In “(…) foot dorsiflexed slightly” we also used the form dorsiflexed (in medical PL: “zgięcie grzbietowe”) because the medical form is understandable, and there is no need to use the colloquial wording (“bend up”) as proposed by the translator as this part of the MNSI is done by professionals.
The same layout as in the document from the University of Michigan was kept, which is similar in the original version but is enriched with a monofilament test. Despite the problem with question 13 in the self-administered part of the MNSI, the differences between the retranslation and original text were related to addressing the patient. For example, in question 1 of the original version, the question was stated as, “Are your legs and/or feet numb?”, whereas the retranslation stated, “Do you experience numbness in your legs and/or feet?” , these differences were deemed to be of little importance.
A study population composed of patients with a diagnosis of DM, using the PL Diabetes Association criteria[6], who attended the NZOZ Nowy Dwor, where the principal researcher works as a diabetologist were identified. The inclusion criteria were: (1) DM diagnosis except gestational; (2) Age ≥ 18; and (3) Native language is PL. Exclusion criteria were: (1) Chronic renal failure requiring renal replacement therapy; (2) Liver cirrhosis; (3) Symptomatic cerebrovascular disease; (4) Active cancer; (5) History of chemotherapy or radiotherapy; (6) Autoimmune disease; (7) Chronic infectious disease; (8) Alcohol or drug abuse; (9) Clinical radicular neuropathy; and (10) Mental or physical conditions that could compromise the evaluation of clinical symptoms. Exclusion criteria were established with a neurologist and were based on prior Portuguese and Turkish validation.
The type of DM, DM duration, body mass, height, body mass index, and glycated hemoglobin no more than 3 mo before the study were collected to characterize the study population. The information necessary to confirm the above-mentioned inclusion and exclusion criteria was obtained from the patient’s records or based on a short interview performed just before the study started if no records were available.
The MNSI with its 2 sections (A and B) was administered to all of the patients twice within an interval of 1-2 wk. A nerve conduction study (NCS) was performed on the same day as the first MNSI. Both specialists who performed the MNSI and NCS were blind to the NCS result and MNSI score for the patient, respectively.
The first part of the MNSI (part A) consists of 15 self-administered “yes/no” questions on foot sensation including pain, numbness, and temperature sensitivity. The scoring is awarded according to a special key. Because items 4 (a circulation measurement) and 10 (a general status measurement) were excluded from the original scale, the highest score for this part of the MNSI is 13 points. The patient should answer all of these questions by him/herself. The higher the points, the more severe the neuropathic symptoms[12].
The next part of the MNSI (part B) is a physical examination involving the assessment of the foot’s appearance (deformities, dry skin, nail abnormalities, callous, infection), a semi-quantitative assessment of vibration sensation at the dorsum of the great toe using a 128-Hz tuning fork, ankle reflexes, and monofilament testing using a 10-g Semmes-Weinstein monofilament. In the original version of the MNSI[11], testing with a monofilament was not included but since cross-sectional studies found it to be highly sensitive for predicting the risk of foot ulceration, it was incorporated into the scale from the University of Michigan[15]. In part B, the results were scored with points assigned separately to positive tests of the right/left foot. The maximum possible score in the second section after adding the monofilament test was 10 points. The information on how to perform tests was incorporated as part of instruction for practitioners and was translated into PL to standardize the examination in Supplementary material (No 1 “Instruction of MNSI-PL for practitioners”).
After permission to validate, we used the English version which is freely available on the MDRC’s website. This MNSI document was translated into PL independently by a diabetologist (being aware of the concept, T1) and a professional translator (not informed of the concept, T2) from GROY in Supplementary material (No 2 “MNSI-PL”). These translations were then compared by a panel of four investigators to identify potential questions about the meaning of items in both sections. Additionally, four randomly selected patients assessed whether the text from the first section was understandable and helped select the more understandable wording among the versions from the translator and the diabetologist where the translation differed. Finally, the translated document was improved (final version – T1/2) and was given to a different professional translator from GROY (BT1) and one professional (BT2) to translate back into English. These individuals were blinded to the original version of the MNSI. Both English versions (retranslated and T1/2) were then compared and discrepancies were identified, reviewed, and confirmed to be acceptable (no conceptual errors were found) by the investigators.
The Viking Quest version 10.0 device connected to a Thermal Sensory Analyzer II 2001 (TSA II), a VSA – 3000 Vibratory Sensory Analyzer (Medoc, Israel), Viking Select version 7.1.1c., and a Nicolet Biomedical device with the Multi-Mode Program (MMP Plus) software were used during the electrophysiological studies. The standard electrophysiological examination consisted of sensory and motor conduction velocity tests with the F-wave estimation. The tests were performed in the left peroneal and tibial nerves and both sural nerves. We assessed distal latency or the shortest latency of F-wave (in milliseconds – ms), potential amplitude (in microvolts – uV for sensory testing, and in millivolts – mV for motor testing), and conduction velocity (in meters per second – m/s). A standard distance between electrodes and distal points of stimulations was preserved for all tests. We used an antidromic technique for sensory conduction velocity estimations. The same conditions were kept during the studies including a room temperature between 21-23 °C and a foot temperature not less than 32 °C[16,17]. Additionally, a conduction velocity distribution test using the collision technique was performed on the peroneal nerve[18]. Vibratory, temperature, and warm- and cold-induced pain thresholds were assessed over the S1 dermatome using quantitative sensory testing[18].
A qualified neurologist obtained the results, if there was an abnormality in any evaluated parameter (latency, amplitude, conduction velocity) in individual nerves in the test, the patient received 1 point. Points 4 meant severe polyneuropathy, 0 normal, 1 minimal, 2 mild, and 3 moderate polyneuropathy.
The scoring for the detection of neuropathy based on NCS for statistical analysis (dichotomous data) was defined as follows: (1) No neuropathy – if no disturbances in nerve conduction were found; and (2) neuropathy – if pathology was found within at least one test. We did not score the severity of neuropathy for statistical analysis.
Statistical analyses were carried out using the computer program Statistics version 14.0 and R version 4.0.3. To statistically evaluate the degree of correspondence between the MNSI and NCS (gold standard) scores, summed values obtained from MNSI parts A and B at both time points, the summed NCS and NCS presented in dichotomous form were used (vide Methodology, NCS). The basis for the estimation of the minimum sample size was the analysis of the reliability of the test with Cronbach’s alpha coefficient[19]. The basis for its estimation was the following accepted assumptions: Minimum acceptable Cronbach’s alpha (H0) = 0.64, expected Cronbach’s alpha (H1) = 0.77, significance level (α) = 0.05; power (1 – β) = 0.8, number of items (k) = 15-20. Based on the accepted assumptions and literature information, the minimum sample size was set at 80.
The continuous data were expressed as means with minimum-maximum values and standard deviations. The qualitative data were expressed as percentages. Reliability was measured by Cronbach’s alpha using Kuder-Richardson Formula 20 (KR-20) coefficient. The consistency values for KR-20 were considered enough when 0.6-0.7; good when 0.7-0.8, and very good when > 0.8. KR-20 values over 0.6 indicated that items had integrity and the test was homogenous[20-22]. Also, the split-half reliability, the Gottman split-half and the correlation between the first and second half were assessed. Intraclass correlation coefficient (ICC) values for consistency (ICC consistency) and agreement (ICC agreement) were calculated for a two-way random effects model to evaluate the stability[20]. An ICC above 0.60 was considered a good fit[21].
To evaluate the statistical significance of the correlation between the summary results obtained using the compared tools (MNSI vs NCS), we used a simple linear correlation, multinomial regression, and a Bland-Altman plot to evaluate the agreement between the two different instruments. The next step was to assess the correlation between the results of the NCS (expressed on a dichotomous scale) and the sum values obtained from parts A and B of the MNSI at the two time points. For this purpose, a multinomial logistic regression model was used. The area under the receiver-operating characteristic (ROC) curves were estimated to measure the discriminatory power of the test. The area under the ROC curve (AUC) represented the performance of a test in predicting the outcome.
A total of 80 patients participated in our validation study (39 women and 41 men; 48.75% and 51.25%, respectively). The sample characteristics are presented in Table 1. Most of the patients were diagnosed with T2DM (n = 77; 96.25%), and only 3 (3.75%) of them had T1DM. The weight and height were the only parameters that differentiated women and men in the studied population. All the patients were tested using the MNSI scale twice (A1B1 and A2B2) and agreed for NCS.
| Variable, n = 80 | Sex | Descriptive statistics | Mann-Whitney U test | ||||
| Valid (n) | Mean | Minimum | Maximum | SD | Pvalue | ||
| Age (yr) | Female | 39 | 69.00 | 45.00 | 80.00 | 6.27 | 0.151516 |
| Male | 41 | 65.32 | 37.00 | 76.00 | 9.56 | ||
| Weight (kg) | Female | 39 | 77.36 | 54.00 | 108.00 | 12.24 | 2.55E-03 |
| Male | 41 | 88.66 | 63.00 | 150.00 | 16.52 | ||
| Height (cm) | Female | 39 | 161.38 | 150.00 | 174.00 | 5.97 | 1.78E-11 |
| Male | 41 | 175.15 | 164.00 | 190.00 | 6.90 | ||
| BMI (kg/m2) | Female | 39 | 29.68 | 21.90 | 40.20 | 4.52 | 2.64E-01 |
| Male | 41 | 28.84 | 21.50 | 44.30 | 4.90 | ||
| HbA1c (%) | Female | 38 | 6.42 | 5.20 | 8.50 | 0.79 | 4.87E-01 |
| Male | 40 | 6.58 | 5.00 | 8.80 | 0.97 | ||
| DM1 duration (yr) | Female | 39 | 12.10 | 0.00 | 37.00 | 8.13 | 2.90E-01 |
The consistency values for KR-20 were considered enough when 0.6-0.7; good when 0.7-0.8, and very good when > 0.8. In our study, the reliability was measured for both parts (A and B) of the MNSI together and separately for A and B. These results showed very good consistency with a Cronbach’s alpha for the full scale of 0.81 for part A and 0.87 for part B (Table 2). The analyses of separated items (A and B) are presented in the Supplementary materials (Supplementary Tables 1 and 2 for items from parts A and B, respectively). KR-20 values over 0.6 indicated that items had integrity, and the test was homogenous which was achieved in all the analyses performed in our study[21,22] (Table 2).
| Part A of MNSI | Part B of MNSI | |||
| n = 80 | Cronbach alpha, full scale: 0.81790 | Cronbach alpha, full scale: 0.87095 | ||
| Corr 1st & 2nd half: 0.745791 | Corr 1st & 2nd half: 0.972206 | |||
| Split-half reliability: 0.854388 | Split-half reliability: 0.985907 | |||
| Guttman split-half: 0.840785 | Guttman split-half: 0.985838 | |||
| Summary for A1 | Summary for A2 | Summary for B1 | Summary for B2 | |
| Mean | 4.250000 | 4.150000 | 6.212500 | 6.175000 |
| Sum | 340.0000 | 332.0000 | 497.0000 | 494.0000 |
| SD | 3.403275 | 4.313946 | 3.925553 | 3.860790 |
| Variance | 11.58228 | 18.61013 | 15.40997 | 14.90570 |
| Cronbach alpha | 0.6499672 | 0.7060083 | 0.7337023 | 0.7346509 |
Regarding stability, all patients were retested within 7-14 d, and the MNSI scores between the test and re-test showed an ICC 0.73 for part A and an ICC 0.97 for part B. For both agreement and consistency, a high level of statistical significance was seen (Table 3). An ICC above 0.60 was considered a good fit[21]. Detailed factor analysis is shown in the Supplementary Tables 3 and 4.
| Part of MNSI | Mean (SD) | ICC for agreement | ICC for consistency | |||
| Test score | Re-test score | ICCagreement | Pvalue | ICCconsistency | Pvalue | |
| A | 2.125 (1.70) | 2.075 (2.16) | 0.728 | 9.05E-15 | 0.725 | 9.47E-15 |
| B | 3.106 (1.96) | 3.088 (1.93) | 0.972 | 6.19E-52 | 0.972 | 8.53E-52 |
The simple linear correlation between the sum of the NCS scores and B scores on the MNSI was statistically significant when comparing the test and re-test (P < 0.001; P < 0.001 respectively). For part A of the MNSI, a statistically significant correlation was confirmed for part A1 (P < 0.001) but not A2 (P > 0.001). Statistically significant bivariate regression models were established for the two time points, confirming a statistically significant correlation of part B and no statistically significant correlation for part A of the MNSI (Supplementary Table 5).
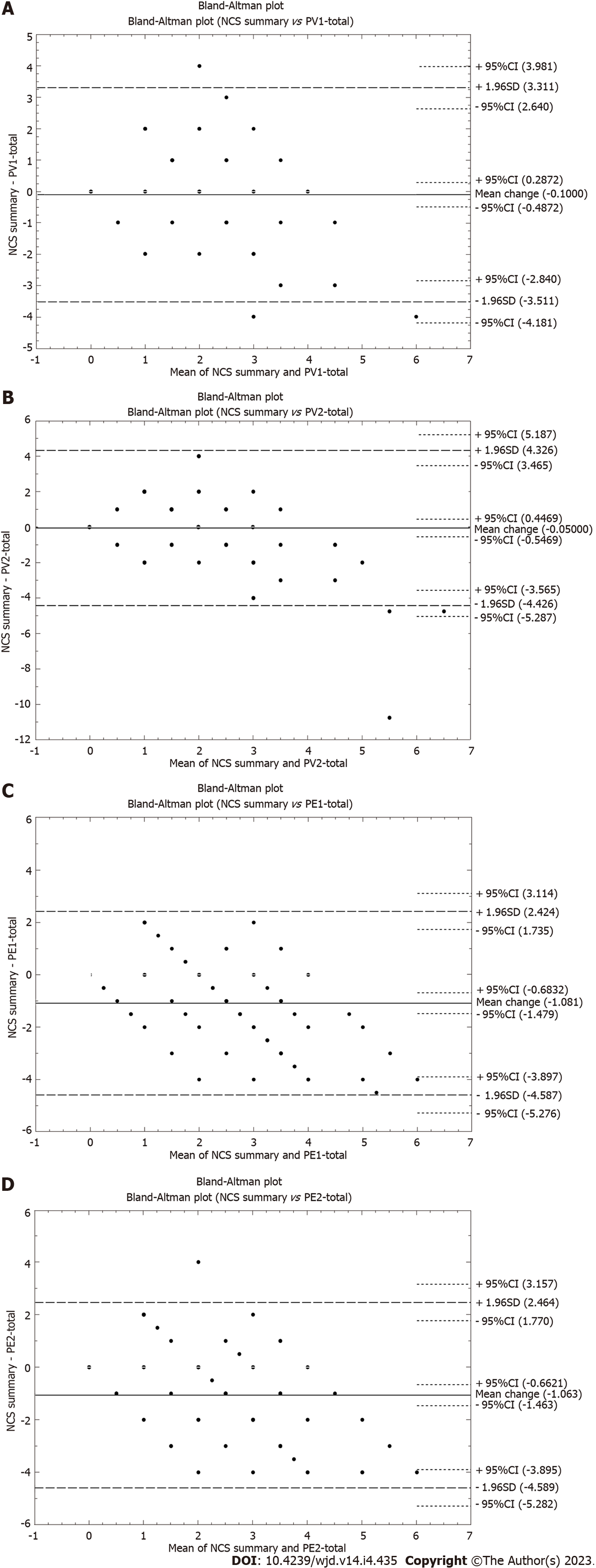
The model of multinomial logistic regression of both parameters (A and B) at time point one [odds ratio (OR) = 2.4, P = 0.022 and OR = 2.3, P = 0.009 for A1 and B1, respectively] and statistical significance for B2 (OR = 1.8, P = 0.009) and its absence for A2 (OR = 1, P = 1) (Supplementary Table 6) were confirmed, thereby also underlining the greater influence of the B-MNSI variable on the occurrence of an event (here neuropathy) assessed using the gold standard NCS. Analysis of the Bland-Altman plots showed high mutual compatibility between the results of the compared tools (MNSI vs NCS, Figure 1).
The ROC area between the NCS and MNSI (section A and/or B) scores helped to validate the scale. A value of 0.70-0.79 indicated reasonable test performance, 0.80-0.89 indicated good performance, and ≥ 0.9 indicated very good performance. The results of AUC for the NCS and both parts of the MNSI (Table 4) reached statistical significance for B1, B2, and A1 (P < 0.001) but not for A2. We determined the cut-off points for the patient’s and practitioner’s part regarding the sensitivity and specificity of each item to predict neuropathy as confirmed by NCS.
| Variable | AUC | Pvalue |
| A1 | 0.749 | 0.0003 |
| B1 | 0.796 | 0.0000 |
| A2 | 0.617 | 0.2296 |
| B2 | 0.770 | 0.0001 |
We obtained the same values for optimal cut-off points during both time points for the MNSI’s patient version (part A) as well as the practitioner version (part B), which could be found as 3 and 2 respectively (Table 5). The sensitivity and specificity cut-off points for A were 33%-40% and 90%-100%, respectively. The sensitivity and specificity cut-off points for B were 81%-84% and 60%-70%, res-pectively.
| Variable | True positives, n, % | False positives, n, % | False negatives, n, % | True negatives, n, % | Sensitivity | Specificity | Optimal cut-off point |
| A1 | 28/35 | 0/0 | 42/52.5 | 10/12.5 | 0.400 | 1.000 | 3 |
| B1 | 57/71.25 | 3/3.75 | 13/16.25 | 7/8.75 | 0.814 | 0.700 | 2 |
| A2 | 23/28.75 | 1/1.25 | 47/58.75 | 9/11.25 | 0.329 | 0.900 | 3 |
| B2 | 59/73.75 | 4/5 | 11/13.75 | 6/7.5 | 0.843 | 0.600 | 2 |
Dedicated to different populations (language, culture differences), the existing instruments in medicine should not only be simply translated but also validated[23]. The validation process should be completed using a representative sample to demonstrate adequate reliability and validity. Peripheral neuropathy is the most common form of diabetic neuropathy, affecting the nerves of the limbs, particularly those of the feet. The MNSI is a low-cost, rapid tool with a user-friendly format that helps detect pathology of the peripheral nervous system in patients with DM and has been validated using NCS (gold standard) for neuropathy detection[15]. After publication, the MNSI has been translated into several languages. A list of available translations that have been validated is available for distribution on the Mapi Research Trust website. However, a PL version has not been added to this list as it has not been validated. Therefore, in our study, we evaluated both the reliability and validity of a PL version of the MNSI following the principles of proper validation[23].
Based on the KR-20, split-half analysis, and ICC, we found that the MNSI-PL is a reliable and stable tool with high internal consistency using a cut-off value of 3 for the questionnaire (part A) and 2 for the examination (part B). The models of multinomial regression and multinomial logistic regression confirmed the greater influence of the B-MNSI variable on the diagnosis of an event (neuropathy) when NCS was used as the gold standard for this diagnosis. The ability to predict neuropathy based on section B was found to be high in other studies[15,24] and even higher compared to section A alone if this part was used together with B[24]. This is understandable as the MNSI consists of two parts, section A which is rather subjective because it is self-administered by the patient, and section B which includes tests to be performed by professionals.
The evaluation of a patient’s peripheral nerve function can be done directly, without subjective bias, by electromyography or biopsy[25-27]. However, because of their invasiveness, persistent discomfort, sensory loss, risk of non-healing, and need to be completed by a specialist, these tools are not first-line diagnostic procedures. The analysis of the Bland-Altman plots in our study unequivocally demonstrates the high concordance between MNSI and NCS test results. This makes the MNSI-PL a reliable screening tool for the diagnosis of peripheral neuropathy in diabetic patients.
For each of the MNSI parts, the cutoff point to confirm neuropathy has been discussed in the literature. However, a cut-off point of 7 for the first section (A in our study) has been previously accepted to be abnormal[11]. Later studies have proposed a cut-off point of ≥ 4 to improve the performance of the MNSI and achieve acceptable levels of sensitivity and specificity. The study that revised the previously existed threshold of ≥ 7 for part A of the MNSI by setting it at ≥ 4 achieved a sensitivity of 40% and specificity of 92%[28]. In our study, a threshold of 3 for part A was found to be the optimal cut-off point and had a specificity of 33%-40%, which shows the probability of having a normal MNSI result in the absence of confirmed neuropathy. The sensitivity reached 90%-100%, which shows the probability of having a positive result in the presence of confirmed neuropathy. Compared to the study by Herman et al[28], we achieved a lower specificity but higher sensitivity and the sensitivity and specificity from our validation study were closer to the results found in the Portuguese[14], Arabic[29] and Turkish[24] analyses. We also proposed a similar cut-off point for A as used in these two studies[14,24]. A high sensitivity gives the test a high ability to correctly identify patients with the disease. Based on our observations during the application of the test to patients, the self-administered part should be filled out with an accompanied professional (doctor, nurse, physiotherapist) to explain any doubts if necessary. It would also be useful when illiterate individuals are completing the questionnaire[24].
For part B (examination) of the MNSI, a score greater than 2 was considered abnormal according to the original scoring algorithm[11]. A threshold of ≥ 2.5 was shown to be 61% sensitive and 79% specific in confirming clinical neuropathy in Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications[27]. However, in the study by Moghtaderi et al[15], the sensitivity and specificity of the examination with a cut-off point of 2 was found to be 65% and 83%. In our study, we found the optimal cut-off point of 2 for section B with a sensitivity of 81%-84% and specificity of 60%-70%, which was similar to the Portuguese study (86% sensitivity and 61% specificity using the same cut-off point)[14].
To summarize both parts of the MNSI, the same cut-off points used in our study for sections A and B were proposed by the authors of the Portuguese version[14] with similar levels of sensitivity and specificity and in one of the Turkish validation studies[30]. As we mentioned, the results from our validation process stressed the importance of part B in detecting neuropathy compared to part A, which was similar to the results of the Turkish validation study[24].
The strengths of our study include a sufficient number of individuals based on the described calculation model, the completion of NCS on the whole group of studied patients, a wide panel of statistical analysis to confirm good reliability and stability of the tool, and confirming the cut-off points for parts A and B seen in previous studies with similar sensitivities and specificities[15,21,28,30,31].
There are limitations to our study. The questionnaire and examination process were limited in which items could not rule out subclinical neuropathy and an autonomic component[32]. Although a small percentage of patients with T1DM (less than 4%) can be considered a limitation because it does not reflect the proportion of different DM types in the population[33], this does not affect the usefulness of the MNSI. Patients with T1DM are usually younger, so we expect that it is easier for them to understand and answer the questions in part A. Additionally, the type of DM has no impact on part B of the MNSI as this is done by a professional.
The recommendations of the PL Diabetes Association do not propose the use of appropriate scales for assessing peripheral neuropathy[6]. This is due to the lack of adaptation of scales such as the MNSI to the PL system, which contributes to a lack of uniformity in assessing a patient’s condition. As a result, some centers create informal evaluation criteria and rules (even though they use the same tools and tests). Similar problems also arise during the design of clinical trials, for which only the English-language scale can be used. Therefore, the introduction of a validated scale would help to standardize screening for neuropathy in diabetic patients.
The MNSI-PL is a reliable screening tool for diabetic peripheral sensorimotor neuropathy when using a cut-off of ≥ 3 for section A and ≥ 2 for section B. Part B is stronger in detecting neuropathy as it is more objective. Additionally, the whole test is useful in clinical practice as well as in our country.
The Michigan Neuropathy Screening Instrument (MNSI) is a collective tool for assessing the peripheral nervous system in patients with diabetes mellitus and is widely used to evaluate patients in many countries.
In Poland, the MNSI has not yet been validated, thus this is a problem when using it in daily practice and for research purposes.
This study aimed to validate both sections (A and B) of the MNSI in Polish (PL) patients with diabetes.
A cross-sectional study using a test (A1, B1) and re-test (A2, B2) formula was performed in 80 patients with diabetes. The gold standard used for neuropathy detection was a nerve conduction study (NCS) which was performed on all participants. Reliability of the MNSI-PL was assessed using the Cronbach’s alpha, Kuder-Richardson formula 20, split-half reliability, the Gottman split-half tests and correlation between first and second half was accessed. Stability was assessed using an intraclass correlation coefficient (ICC). For external validation, we used simple linear correlation, binomial regression, and agreement between two different tools using a Bland-Altman plot analysis.
The scale was internally consistent (Cronbach’s alpha for the full scale: 0.81 for A and 0.87 for B). MNSI-PL scores between test/retest showed high stability (ICC = 0.73 for A and ICC = 0.97 for B). The statistically important correlations between MNSI-PL and NCS were found for B1, B2, and A1 (P < 0.005). The cut-off points of ≥ 3 for section A (sensitivity of 90%-100%; specificity of 33%-40%) and ≥ 2 for section B (sensitivity of 81%-84%; specificity of 60%-70%) were obtained during neuropathy detection.
The MNSI-PL is a reliable and valid instrument in screening for diabetic neuropathy.
PL MNSI is a reliable and accurate screening tool for peripheral neuropathy. We also proposed cutoff points for both scales (for patients and for physicians), and hope that this can also be used by other authors from different countries.
We would like to thank NZOZ Nowy Dwor for agreeing to recruit patients in their clinic.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Fan Z, China; Messias LHD, Slovakia; Ozden F, Turkey; Wan H, China S-Editor: Wang JJ L-Editor: Ma JY-MedE P-Editor: Wang JJ
| 1. | Bakker K, Schaper NC; International Working Group on Diabetic Foot Editorial Board. The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28 Suppl 1:116-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 227] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 3. | Hicks CW, Selvin E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr Diab Rep. 2019;19:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 327] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 4. | Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. 2017;8:646-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 5. | Ibrahim A. IDF Clinical Practice Recommendation on the Diabetic Foot: A guide for healthcare professionals. Diabetes Res Clin Pract. 2017;127:285-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Araszkiewicz A, Badurska-Stankiewicz E, Borys S, Budzyński A, Cyganek K, Cypryk K, Czech A, Czupryniak L, Drzewoski J, Dzida G, Dziedzic T, Franek E, Gajewska D, Gawrecki A, Górska M, Grzeszczak W, Gumprecht J, Idzior-Waluś B, Jarosz-Chobot P, Kalarus Z, Karczewska-Kupczewska M, Klupa T, Koblik T, Kokoszka A, Korzon-Burakowska A, Kowalska I, Krętowski A, Majkowska L, Malecki M, Mamcarz A, Mirkiewicz-Sieradzka B, Młynarski W, Moczulski D. 2021 Guidelines on the management of patients with diabetes. A position of Diabetes Poland. Clin Diabetol. 2021;10:1-113. [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Wood WA, Wood MA, Werter SA, Menn JJ, Hamilton SA, Jacoby R, Dellon AL. Testing for loss of protective sensation in patients with foot ulceration: a cross-sectional study. J Am Podiatr Med Assoc. 2005;95:469-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21:1714-1719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 232] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Armstrong David G. Detection of diabetic peripheral neuropathy: strategies for screening and diagnosis. In: Advanced Studies in Medicine. Somerville: Galen Publishing, 2005; 5: S1033-S1037. [Cited in This Article: ] |
| 10. | American Diabetes Association Professional Practice Committee; Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S, Leon J, Lyons SK, Peters AL, Prahalad P, Reusch JEB, Young-Hyman D. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S185-S194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 11. | Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 856] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 12. | Lunetta M, Le Moli R, Grasso G, Sangiorgio L. A simplified diagnostic test for ambulatory screening of peripheral diabetic neuropathy. Diabetes Res Clin Pract. 1998;39:165-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Dyck PJ, Karnes J, O'Brien PC, Swanson CJ. Neuropathy Symptom Profile in health, motor neuron disease, diabetic neuropathy, and amyloidosis. Neurology. 1986;36:1300-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Barbosa M, Saavedra A, Severo M, Maier C, Carvalho D. Validation and Reliability of the Portuguese Version of the Michigan Neuropathy Screening Instrument. Pain Pract. 2017;17:514-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108:477-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | OH Shin J. Clinical electromyography: nerve conduction studies. Baltimore: Lippincott Williams & Wilkins, 2003. [Cited in This Article: ] |
| 17. | Mallik A, Weir AI. Nerve conduction studies: essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry. 2005;76 Suppl 2:ii23-ii31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Sander HW. Collision testing. In: Kimura J, Handbook of clinical neurophysiology. United States: Elsevier, 2006; 7: 359-380. [Cited in This Article: ] |
| 19. | Siao P, Cros DP. Quantitative sensory testing. Phys Med Rehabil Clin N Am. 2003;14:261-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Bonett DG. Sample size requirements for testing and estimating coefficient alpha. J Educ Behav Stat. 2002;27:335-340. [DOI] [Cited in This Article: ] |
| 21. | Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5565] [Cited by in F6Publishing: 6755] [Article Influence: 375.3] [Reference Citation Analysis (0)] |
| 22. | Cam MO, Baysan-Arabacı L. Qualitative and quantitative steps on attitude scale construction. Turkish J Res Devel in Nurs. 2010;12:59-71. [Cited in This Article: ] |
| 23. | Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25:3186-3191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6161] [Cited by in F6Publishing: 6900] [Article Influence: 287.5] [Reference Citation Analysis (0)] |
| 24. | Aktar Reyhanioğlu D, Adiyaman SC, Bektaş M, Bulut O, Özgen Saydam B, Bayraktar F, Kara B. Validity and reliability of the Turkish version of the Michigan Neuropathy Screening Instrument. Turk J Med Sci. 2020;50:789-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Theriault M, Dort J, Sutherland G, Zochodne DW. A prospective quantitative study of sensory deficits after whole sural nerve biopsies in diabetic and nondiabetic patients. Surgical approach and the role of collateral sprouting. Neurology. 1998;50:480-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Kennedy WR, Nolano M, Wendelschafer-Crabb G, Johnson TL, Tamura E. A skin blister method to study epidermal nerves in peripheral nerve disease. Muscle Nerve. 1999;22:360-371. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 324] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, Feldman EL; DCCT/EDIC Research Group. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29:937-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 304] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 29. | Abuzinadah AR, Alkully HS, Alanazy MH, Alrawaili MS, Milyani HA, AlAmri B, AlShareef AA, Bamaga AK. Translation, validation, and diagnostic accuracy of the Arabic version of the Michigan neuropathy screening instrument. Medicine (Baltimore). 2021;100:e27627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 30. | Kaymaz S, Alkan H, Karasu U, Çobankara V. Turkish version of the Michigan Neuropathy Screening Instrument in the assessment of diabetic peripheral neuropathy: a validity and reliability study. Diabetol Int. 2020;11:283-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Xiong Q, Lu B, Ye H, Wu X, Zhang T, Li Y. The Diagnostic Value of Neuropathy Symptom and Change Score, Neuropathy Impairment Score and Michigan Neuropathy Screening Instrument for Diabetic Peripheral Neuropathy. Eur Neurol. 2015;74:323-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed). 1982;285:916-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 832] [Cited by in F6Publishing: 828] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 33. | International Diabetes Federation. Type 1 diabetes. [cited 3 July 2022]. Available form: https://www.idf.org/aboutdiabetes/type-1-diabetes.html. [Cited in This Article: ] |