Published online Apr 15, 2023. doi: 10.4239/wjd.v14.i4.352
Peer-review started: November 27, 2022
First decision: December 26, 2022
Revised: January 10, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: April 15, 2023
Processing time: 136 Days and 2 Hours
Insulin resistance is a condition in which the target tissues have a decreased response to insulin signaling, resulting in glucose uptake defect, and an increased blood sugar level. Pancreatic beta cells thus enhance insulin production to compensate. This situation may cause further beta cell dysfunction and failure, which can lead diabetes mellitus (DM). Insulin resistance is thus an important cause of the development of type 2 DM. Insulin resistance has also been found to have a strong relationship with cardiovascular disease and is common in chronic kidney disease (CKD) patients. The mechanisms of insulin resistance in CKD are complex and multifactorial. They include physical inactivity, inflammation and oxidative stress, metabolic acidosis, vitamin D deficiency, adipose tissue dysfun
Core Tip: Insulin resistance is the main cause of type 2 diabetes mellitus and is associated with cardiovascular events. It is also common in chronic kidney disease patients. We discuss the mechanisms of insulin resistance in such patients and the interaction of currently available anti-diabetic medications with insulin resistance.
- Citation: Lin WR, Liu KH, Ling TC, Wang MC, Lin WH. Role of antidiabetic agents in type 2 diabetes patients with chronic kidney disease. World J Diabetes 2023; 14(4): 352-363
- URL: https://www.wjgnet.com/1948-9358/full/v14/i4/352.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i4.352
Insulin resistance is a condition in which a tissue or organ has reduced sensitivity to insulin-initiated biological processes. To compensate for lower sensitivity, insulin secretion by pancreatic beta cells increases, causing hyperinsulinemia. Insulin resistance is thought to be an important contributor to beta cell dysfunction, which eventually leads to diabetes mellitus (DM). It is associated with risk factors for cardiovascular (CV) disease, such as inflammation, oxidative stress, and endothelial dysfunction[1]. Diabetes nephropathy is a common complication of DM. It causes albuminuria and renal function deterioration[2]. Insulin resistance is also common in chronic kidney disease (CKD) patients[3]. There are various anti-diabetes medications, including biguanides, sulfonylureas (SUs), thiazolidinediones (TZDs), alfa-glucosidase inhibitors (AGIs), glucagon-like peptide-1 (GLP-1)-based agents, and sodium-glucose co-transporter-2 inhibitors (SGLT2Is). In this article, we briefly summarize the mechanism of insulin resistance in CKD patients and the effect of currently available anti-diabetic medications on insulin resistance.
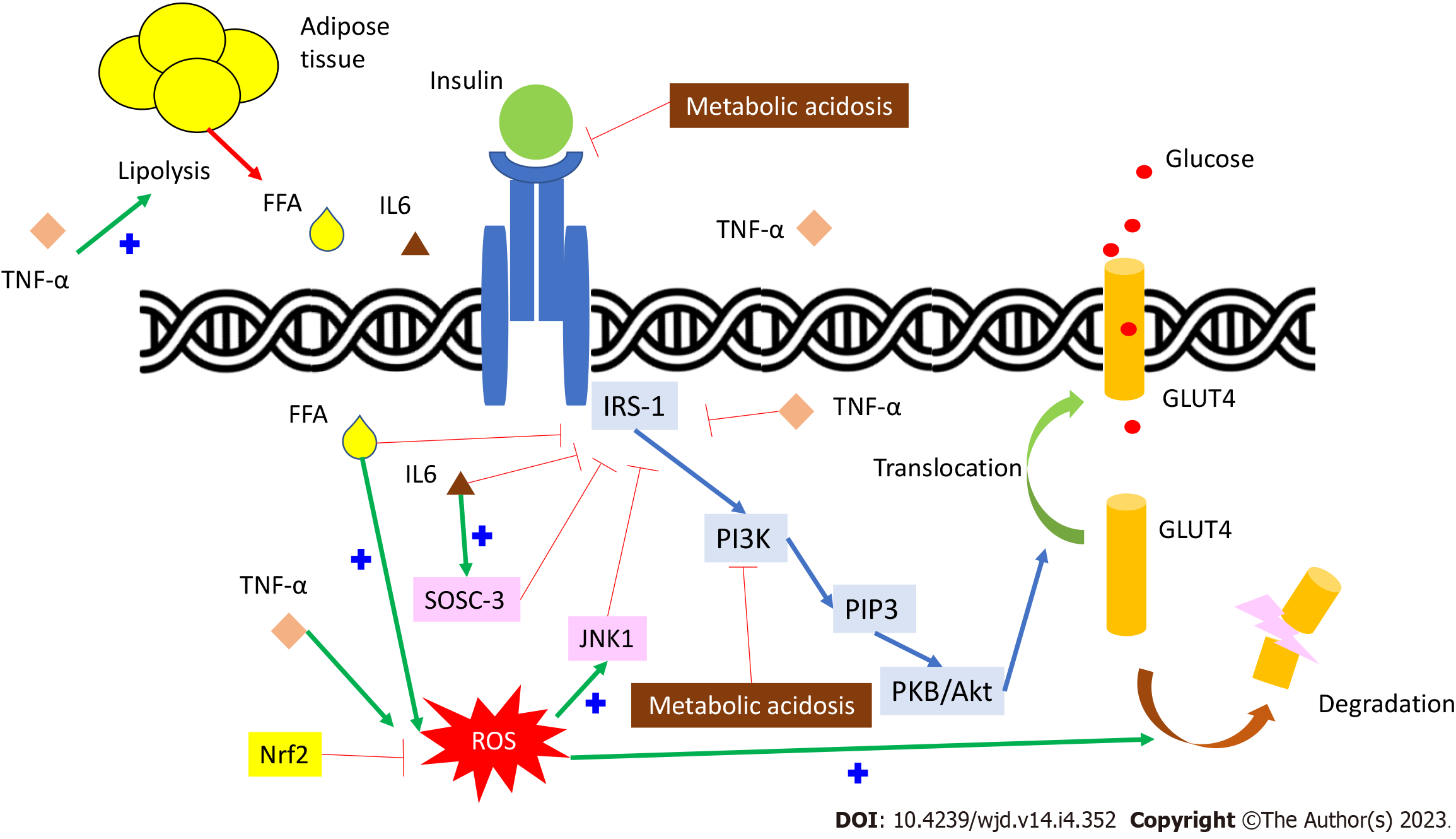
Insulin binds to its receptor and induces insulin receptor substrate-1 (IRS-1) tyrosine phosphorylation. IRS-1 then phosphorylates phosphatidyl-inositol-3-kinase and produces phosphatidylinositol-triphosphate (PIP3). PIP3 activates the protein kinase B/Akt pathway[1] (Figure 1). This effect can induce glucose transporter 4 (GLUT4) translocation to the cell membrane and cause glucose uptake. Insulin resistance presents when this signaling pathway dysfunction occurs. There are many different effects on the insulin signaling pathway in CKD patients, which subsequentially cause insulin resistance.
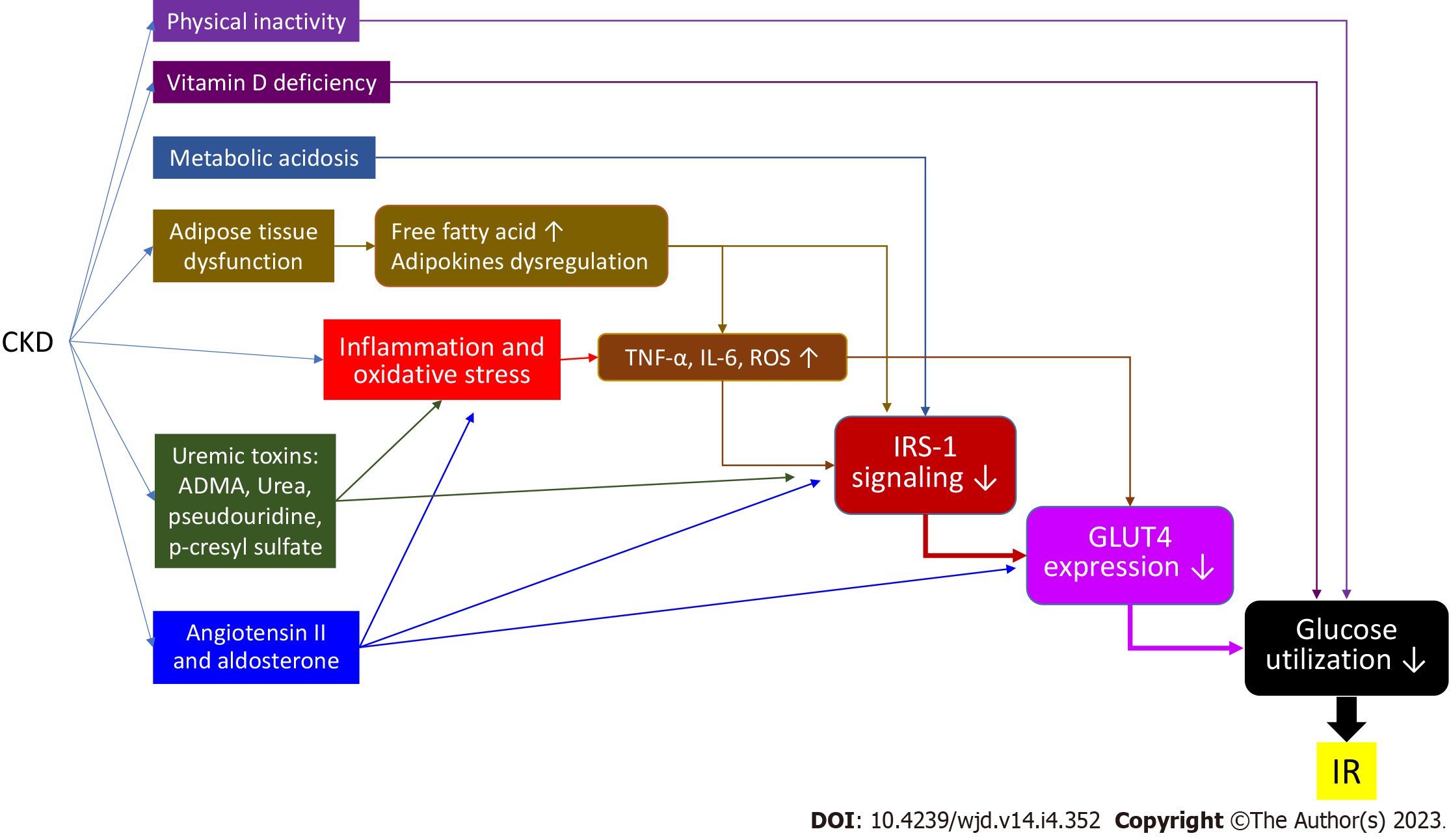
Insulin resistance may increase after several days of bed rest in a healthy population[4] and contributes to impaired microvascular function. Physical activity is decreased in CKD patients[1]. In the CKD mouse model, more physical activity can increase insulin sensitivity[5]. In patients with end-stage kidney disease (ESRD), moderate physical training can improve glucose tolerance and reduce the plasma insulin level[6].
Serum proinflammatory cytokines, such as C-reactive protein, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), are elevated in CKD patients, indicating systemic inflammation and increased oxidative stress[7]. TNF-α can directly inhibit IRS-1 function[8,9] and cause free fatty acid (FFA) accumulation by activating lipolysis, which indirectly inhibits IRS-1[10]. IL-6 can also, directly and indirectly, inhibit IRS-1 by stimulating the suppressor of cytokine signaling-3 pathway[11,12]. Reactive oxygen species (ROS), generated by inflammatory cytokines, FFA oxidation, or mitochondria, not only inhibit IRS-1 phosphorylation but also induce GLUT4 degradation by activating the casein kinase-2 pathway[13]. The expression and function of nuclear factor-erythroid-2-related factor-2 (Nrf2), which can enhance antioxidant and anti-inflammatory activity genes, are reduced in CKD patients[14]. Nrf2 deficiency might be another cause of insulin resistance in CKD patients[1,15] (Figure 1).
Metabolic acidosis is a common metabolic abnormality due to an impairment of renal acid excretion in CKD patients. Metabolic acidosis is also a risk factor for insulin resistance due to impaired glucose metabolism and cellular insulin sensitivity[16]. Metabolic acidosis reduces insulin binding to its receptor IRS-1 and down-regulates the following intracellular signaling in adipocytes and myocytes[17,18] (Figure 1). Furthermore, insulin resistance can be reduced by correcting metabolic acidosis in CKD patients[19].
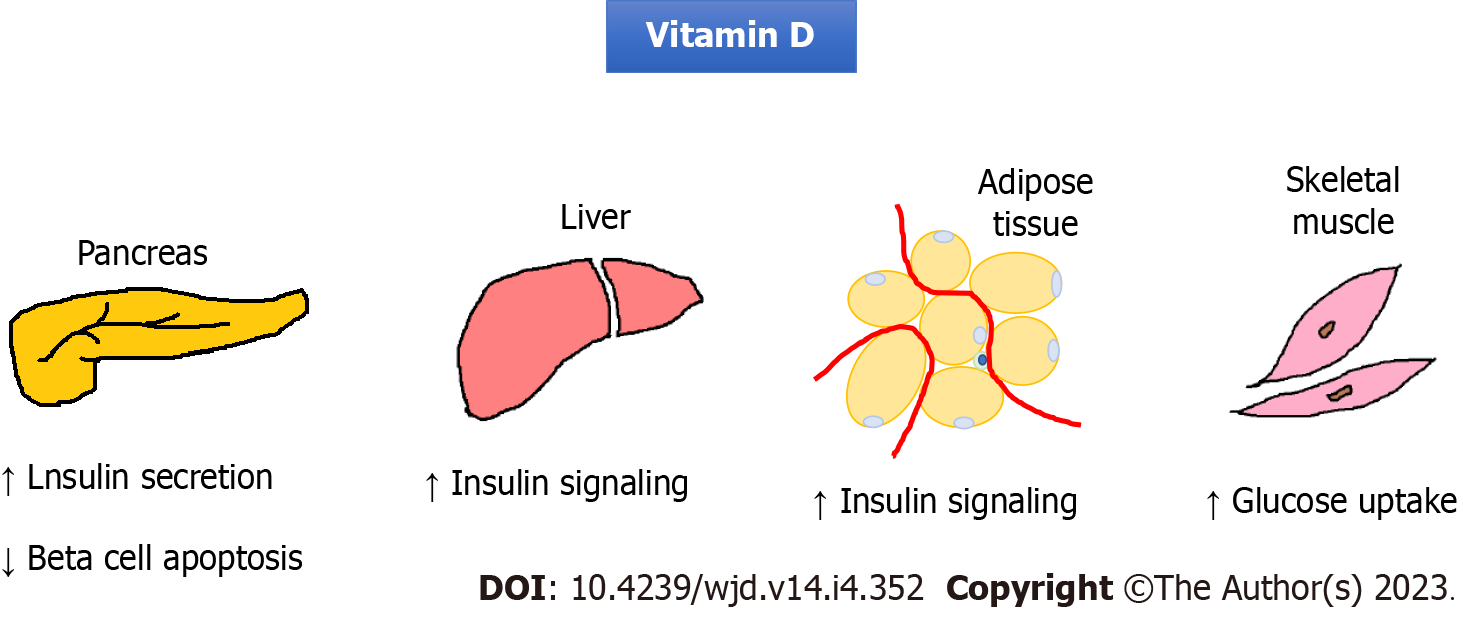
Vitamin D is a hormone that regulates calcium homeostasis. Vitamin D may have a role in insulin secretion because of the vitamin D receptor presenting in pancreatic beta cells[20]. Vitamin D can also reduce pancreatic islets apoptosis caused by systemic chronic inflammation[21]. Insulin secretion by pancreatic beta cells requires extracellular calcium infusion. The vitamin D level is important for the normal homeostasis of extracellular calcium levels[22]. Moreover, vitamin D can promote insulin-induced uptake from the liver, adipose tissue, and skeletal muscle tissue[23] (Figure 2). A large cross-sectional study revealed a strong association between the vitamin D levels and insulin resistance[24]. Some epidemiological studies also showed an association between vitamin D deficiency and risk for type 2 DM (T2DM)[25]. Therefore, many trials have tried to examine the therapeutic potential of vitamin D supplementation. However, a meta-analysis that included 28 randomized controlled trials in 2018 showed that vitamin D supplementation had no significant effect on controlling the fasting plasma glucose level, improving insulin resistance, or preventing T2DM[25]. In contrast, two recent studies showed that high-dose vitamin D supplementation could reduce insulin resistance and oxidative stress[26,27]. Further investigation is needed to clarify the effect of vitamin D on insulin resistance.
Obesity enhances hepatic gluconeogenesis and increases circulating FFAs through lipolysis of adipose tissue. It has been hypothesized that hyperinsulinemia is the initial effect in obese patients[28]. Furthermore, adipose tissue can secret adipokines and inflammatory markers[29]. These conditions can subsequently induce insulin resistance. CKD patients suffer from adipose reduction. However, many metabolic abnormalities present in CKD patients are similar to those in the obese condition, such as a high circulating FFA level and a high level of inflammatory cytokines[30]. Adipose disarrangement and adipokine dysregulation may be the cause[31].
Uremic toxin accumulation may induce insulin resistance in CKD patients. Water-soluble toxins, such as asymmetric dimethylarginine (ADMA)[32] and pseudouridine[33], have been proven to cause insulin resistance. ADMA, an endogenous nitric oxide synthase inhibitor, can cause endothelial dysfunction. It could reduce IRS-1 and GLUT4 expression and induce IRS-1 degradation[34]. Pseudouridine inhibits insulin signaling and glucose uptake in rat muscle cells. Urea can induce ROS generation and cause insulin resistance in the uremic mouse model[35]. Furthermore, a positive relationship between high blood urea nitrogen and incident DM has been reported[36]. The serum level of protein-bound toxins, such as p-cresyl sulfate, also increases in CKD patients. P-cresyl sulfate can cause an impaired insulin signaling pathway in animal models[37]. It also results in lipid redistribution to the liver and muscle, which increases ROS production and inflammation[37].
A high level of angiotensin II (Ang II), which is a common condition in CKD patients, may cause insulin resistance. Ang II stimulates IL-6 production[38], which then causes insulin resistance. Aldosterone increases as renal function declines. Aldosterone can induce insulin resistance not only directly by impairing IRS-1 function and GLU4 translocation, but also indirectly by affecting the production of other circulating factors such as inflammatory cytokines[39]. A small study showed that the administration of a mineralocorticoid receptor antagonist (MRA) such as spironolactone can ameliorate insulin resistance in CKD patients and rats[40]. However, the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity clinical study revealed that steroidal MRAs may increase the risk of new DM development[41]. A recent clinical study showed no improvement in insulin sensitivity in a T2DM patient after 8 wk of steroidal MRA eplerenone use[42]. The benefit of the use of steroidal MRAs in the management of insulin resistance is not clear. Moreover, for CKD patients, steroidal MRAs should be used with caution because of the higher risks of hyperkalemia and gynecomastia (Figure 3).
It is well known that chronic high blood sugar itself can cause insulin resistance and pancreatic islet beta cell dysfunction, which is called glucotoxicity[43]. This effect is mainly through the activation of oxidative stress (as previously described). Therefore, lowering the blood sugar level can attenuate insulin resistance. All anti-diabetic medication decreases the blood sugar level and thus partially decreases insulin resistance. As previously described, high weight and obesity are other common causes of insulin resistance. Therefore, body weight loss is expected to decrease insulin resistance. Some anti-diabetic medications decrease body weight and thus could improve insulin resistance via this mechanism.
Metformin is a first-line drug for treating T2DM[44]. Metformin reduces blood glucose mainly through the suppression of hepatic gluconeogenesis by activating liver AMP-activated protein kinase (AMPK) without causing hypoglycemia[45]. AMPK also reduces liver lipogenesis and increases FFA oxidation, thereby decreasing liver steatosis and increasing hepatic insulin sensitivity[45]. In addition to inhibiting hepatocyte glucose production, metformin enhances insulin-stimulated glucose utilization and FFA oxidation in peripheral tissue, including skeletal muscle and fat tissue[46]. Metformin also stimulates enterocytes to uptake and utilize glucose, which results in a net glucose absorption decrease[45], and induces GLP-1 secretion[45]. Polycystic ovary syndrome (PCOS) is a disease with insulin resistance and hyperinsulinemia. The administration of metformin can improve dyslipidemia and inflammation[47]. However, administration for CKD patients should be done carefully due to its side effect of lactic acidosis. Despite the scarcity of data on the reduction of insulin resistance by metformin in CKD patients, metformin has been shown to slow the progression of DM in high-risk population[48].
TZDs are nuclear transcription factor peroxisome proliferator-activated receptor (PPAR) agonists. PPARγ is essential for new insulin-sensitive adipocyte differentiation and promotes FFA uptake and storage in subcutaneous adipose rather than visceral adipose tissue[49,50]. A reduction in the FFA level is associated with insulin resistance reduction. TZDs also increases glucose uptake by hepatocytes and skeletal muscle cells by increasing GLUT4 expression and translocation[49,50]. These effects decrease serum glucose without elevating insulin, which improves insulin resistance. Some clinical research showed that TZDs could also improve insulin resistance in hemodialysis patients[51,52]. However, side effects associated with subcutaneous adipose deposition (e.g., weight gain) and fluid retention, which can lead to heart failure, limit their clinical use[50]. TZDs may be a good option for sugar control because of the limited choice for T2DM in CKD patients. TZDs have a beneficial effect on reducing insulin resistance but the side effect of fluid retention is of concern.
SUs can increase insulin secretion by altering the resting potential of islet beta cells via the inhibition of the adenosine triphosphate (ATP)-sensitive potassium channel (K-ATP channel). SUs are mainly removed from the liver and kidney, so the effect may be enhanced in CKD patients[53,54]. SUs can enhance peripheral glucose utilization directly by increasing GLUT4 expression in vitro and indirectly due to a reduction of glucotoxicity[55]. The improvement in insulin resistance by SUs may be a short-term effect[56]. Meglitinides can also stimulate insulin secretion via a similar pathway with a shorter onset time and duration. The effect of meglitinides on insulin resistance is still unclear[57]. Meglitinides can reduce glucotoxicity like SUs and temporally improve insulin resistance. However, body weight gain is a common side effect of SUs and meglitinides, which may increase insulin resistance. A recent study examined the effect of meglitinides in hemodialysis patients. Compared to the placebo group (which used only voglibose, an AGI), add-on meglitinide significantly decreased insulin resistance, fasting glucose, hemoglobin A1c (HbA1c) and glycated albumin levels[58]. The decrease in insulin resistance caused by meglitinide may be via a decrease in glucotoxicity.
AGIs can reversibly depress intestinal alpha-glucosidase activity, which delays sugar absorption[59]. They can reduce postprandial hyperglycemia without increasing the insulin level. Acarbose, an alpha-glucosidase inhibitor, was found to improve insulin sensitivity in fructose-fed rats[60]. In the STOP-NIDDM trial, acarbose administration in impaired-glucose-tolerance patients significantly increased the reversion of impaired glucose tolerance to normal glucose tolerance[61] and significantly reduced the risk of CV events and hypertension[62]. Acarbose has a potential side effect of hepatotoxicity that is possibly dose-dependent[63]. Due to accumulation in CKD, acarbose should be avoided in these patients[63]. However, a recent study showed that there was no relationship between acarbose use and liver injury in a severe renal insufficiency group[64]. So far, there is little evidence that acarbose improves the insulin signaling pathway. A study showed that voglibose monotherapy in hemodialysis patients could reduce HbA1c but not clinical insulin resistance[58].
GLP-1, which is secreted from the small intestine, can stimulate insulin secretion from pancreatic islet beta cells after food intake. It can also delay gastric emptying, inhibit inappropriate post-meal glucagon release, and decrease appetite via the central nervous system[65,66]. GLP-1 is rapidly switched to an inactive form by dipeptidyl peptidase-4 (DPP-4) enzyme[67]. Therefore, DPP-4 inhibitors and GLP-1 agonists can both decrease the post-prandial blood sugar level without obvious hypoglycemia.
DPP-4 inhibitors have a neutral effect on CV events, development or progression of renal function, and body weight[68-71]. Some studies showed that DPP-4 inhibitors, such as sitagliptin[72] and vildagliptin[73], can improve clinical insulin sensitivity. Some studies also revealed that DPP-4 inhibitors have an anti-inflammation effect[74,75]. A 2019 meta-analysis showed that DPP-4 inhibitors can improve both beta cell function and insulin resistance, although the effect was weak[76]. A new DPP-4 inhibitor omarigliptin was found to reduce insulin resistance and systemic inflammation in T2DM patients[77]. However, there is current no evidence that the use of DPP4-inhibitors reduces insulin resistance in the CKD group.
GLP-1 receptor agonists (GLP-1RAs) have been shown to have benefits for atherosclerotic CV disease (ASCVD) and have been suggested as a first-line therapy for patients with ASCVD or a high risk of ASCVD[44]. GLP-1RAs can attenuate oxidative stress, ameliorate inflammatory response, increase GLUT4 expression and translocation, amplify insulin signaling transduction, and improve the plasma lipid profile[78]. All of these effects can improve insulin sensitivity. GLP-1RAs also have an effect on body weight loss[79,80], which also decreases insulin resistance. The meta-analysis in 2019 also showed that GLP-1RAs significantly increase islet beta cell function and reduce insulin resistance and the fasting glucose level[76]. Furthermore, GLP-1RAs are a potential treatment choice for PCOS, which is highly correlated with insulin resistance[81]. GLP-1RAs such as liraglutide, dulaglutide and semaglutide, have been reported to have good efficacy and a good safety profile in the advanced CKD group, including hemodialysis patients[82]. Although there is currently no direct evidence of GLP-1RAs improving insulin resistance in the CKD group, it can be hypothesized theoretically.
SGLT2Is inhibit renal tubule glucose reabsorption, and thus increase urinary glucose excretion and improve hyperglycemia. SGLT2Is also have a diuretic effect by decreasing sodium reabsorption. SGLT2Is have favorable CV effects, especially in the heart failure group[44]. SGLT2Is can delay renal function deterioration with or without DM[83-85]. The effect may result from decreasing intra-glomerular pressure via tubuloglomerular feedback. Some studies have recently shown that SGLT2Is also have potential for improving insulin sensitivity. Tofogliflozin, a class of SGLT2I, was found to improve insulin resistance in skeletal muscle by stimulating glucose uptake in obese mice[86]. It also accelerated lipolysis in adipose tissue and reduced adipose tissue mass. The reduction of hyperinsulinemia as a result of decreasing blood sugar level after the administration of SGLT2Is may be the mechanism. Another SGLT2I, empagliflozin, can reduce fat mass by increasing energy expenditure, promoting fat browning, and enhancing fatty acid oxidation in skeletal muscle in high-fat-diet-induced obese mice[87]. It was also found to induce M2 macrophage polarization in fat and the liver, which had an anti-inflammation effect[87]. In humans, dapagliflozin can reduce body weight and body fat mass and improve muscle insulin sensitivity[88]. In summary, SGLT2Is can stimulate glucose uptake in skeletal muscle tissue, decrease fat mass, promote fat browning, and reduce inflammation. These effects can attenuate insulin resistance. However, these effects are decreased in CKD patients. The effect of SGLT2Is on insulin resistance in the CKD group is unclear (Table 1).
| Insulin resistance | CV effects[44] | CKD group | ||
| ASCVD | HF | |||
| Metformin | Liver gluconeogenesis (increased). Peripheral tissue glucose utilizing (increased). Net glucose absorption (decreased). Insulin resistance (markedly decreased) may benefit PCOS | Potential benefit | Neutral | eGFR < 30: Lactic acidosis |
| TZDs | Insulin signaling (increased). FFA (increased). Insulin resistance (markedly decreased) | Potential benefit | Increase risk | No dose adjustment required. Fluid retention |
| SUs and meglitinides | Glucotoxicity (decreased). GLUT4 expression (increased). Insulin resistance, mainly by decreasing glucotoxicity (decreased) | Neutral | Neutral | Low dose initiation to prevent hypoglycemia |
| Alpha-glucosidase inhibitors | Glucotoxicity (decreased). Insulin resistance, mainly by decreasing glucotoxicity (decreased) | Potential benefit | Neutral | Contraindication in CrCl < 25 (lack of data). Liver injury? |
| DPP-4 inhibitors | Improved islet beta cell function. Inflammation (decreased)? Insulin resistance (decreased) | Neutral | Potential risk: Saxagliptin and alogliptin | Generally safe. They can be used in CKD group (no dose adjustment for linagliptin) |
| GLP-1RAs | Oxidative stress, inflammation (decreased). GLUT4 expression (increased). Insulin signaling (increased). Body weight (decreased): Insulin resistance (markedly decreased) | Benefit | Neutral | No dose adjustment required |
| SGLT2Is | Peripheral tissue glucose utilization (increased). Energy expenditure (increased). Induce M2 macrophage polarization: Insulin resistance (markedly decreased) | Benefit | Benefit | Decrease sugar lowering effect in CKD group. Do not initiate when eGFR < 20 |
As mentioned, protein-bound uremic toxins such as p-cresyl sulfate can induce insulin resistance. AST-120 (Kremezin) is an oral carbonaceous adsorbent. It can absorb toxins generated by intestinal microbiota and decrease the systemic and local uremic toxin levels. A study compared AST-120-fed diabetic CKD (underwent two-third nephrectomy) rats to control diabetic CKD rats[89]. The mean blood glucose level and the mean dose of exogenous insulin used in the AST-120-fed group were significantly reduced[89].
As previously described, Ang II can induce insulin resistance. The main pathway for producing Ang II is via the renin-Ang system (RAS). The use of RAS blockades, including Ang-converting-enzyme inhibitor and Ang II receptor blocker, are standard care for CKD and diabetes nephropathy because of their beneficial effects on CV and kidney outcomes. Some studies showed that RAS blockades improve insulin resistance compared to other anti-hypertensive agents[90,91]. A large study that recruited 5269 impaired-glucose-tolerance patients showed that the administration of ramipril significantly reduced the post-load serum glucose level[92]. Another large trial showed that the use of valsartan decreased serum fasting glucose, the post-load glucose level, and even 5-year diabetes development[93].
Aldosterone overproduction may induce insulin resistance. The current evidence for the benefit of using steroidal MRAs to manage insulin resistance is still unclear. Finerenone, a novel non-steroidal MRA, has a higher affinity to the mineralocorticoid receptor and fewer side effects compared to those of steroidal MRAs. Recent studies indicated that finerenone has a beneficial effect on the heart and kidneys in CKD and T2DM patients[94,95]. A recent animal study found that finerenone can increase brown adipose tissue and thus improve insulin resistance[96]. Finerenone may be an attractive therapy choice for treating insulin resistance in CKD patients.
Endothelin-1 (ET-1) is a peptide secreted by endothelium. ET-1 is thought to induce vasoconstriction, cell proliferation, and inflammation. There is a positive correlation between the serum ET-1 level and insulin resistance[97]. The administration of an endothelin receptor antagonist, such as atrasentan, can improve hepatic insulin sensitivity in insulin resistance rats. A recent study revealed that atrasentan can improve peripheral glucose homeostasis, dyslipidemia, and liver triglycerides in high-fat-diet mice[98]. Therefore, ET-1 antagonists may be a therapeutic choice for improving insulin resistance. The SONAR trial showed better kidney outcomes with long-term atrasentan use in selected T2DM patients with CKD. However, fluid overload and anemia were significantly higher in the atrasentan group[99].
CKD patients have a higher risk for CV events. Insulin resistance is common in CKD patients and may cause CV disease in this group. Therefore, knowing the pathogenesis of insulin resistance in CKD is crucial. It can provide a way to manage potential CV comorbidities or ESRD. Several kinds of anti-diabetic medication can not only decrease the blood sugar level but also improve insulin resistance. Some of them may have a beneficial effect on CKD. Moreover, recent novel medications for managing CKD can also reduce insulin resistance. MicroRNAs (miRNAs) are small non-coding RNAs that can regulate target mRNA expression[100]. They have been found to have an effect on glucose metabolism. For example, a recent study showed that miR-126 single nucleotide polymorphism was associated with T2DM[101]. MiRNAs may be a target therapeutic strategy in the future. Small-molecule insulin mimetics, which can stimulate the insulin-signaling pathway, have been recently introduced[102,103]; however, this research is still in the early stages. Further research on halting insulin resistance in CKD patients is required.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balbaa ME, Egypt; Elfaki I, Saudi Arabia; Hojs R, Slovenia S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311:F1087-F1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 2. | Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1-S127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 366] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 3. | Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, Ritz E. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53:1343-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF Jr, Vita JA. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650-2656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 289] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Davis TA, Klahr S, Karl IE. Glucose metabolism in muscle of sedentary and exercised rats with azotemia. Am J Physiol. 1987;252:F138-F145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Goldberg AP, Hagberg JM, Delmez JA, Haynes ME, Harter HR. Metabolic effects of exercise training in hemodialysis patients. Kidney Int. 1980;18:754-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 81] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Liao MT, Sung CC, Hung KC, Wu CC, Lo L, Lu KC. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol. 2012;2012:691369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Lee DF, Kuo HP, Chen CT, Wei Y, Chou CK, Hung JY, Yen CJ, Hung MC. IKKbeta suppression of TSC1 function links the mTOR pathway with insulin resistance. Int J Mol Med. 2008;22:633-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939-2945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 441] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 10. | Plomgaard P, Fischer CP, Ibfelt T, Pedersen BK, van Hall G. Tumor necrosis factor-alpha modulates human in vivo lipolysis. J Clin Endocrinol Metab. 2008;93:543-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391-3399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 613] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 12. | Rieusset J, Bouzakri K, Chevillotte E, Ricard N, Jacquet D, Bastard JP, Laville M, Vidal H. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes. 2004;53:2232-2241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biomed J. 2017;40:257-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 14. | Aminzadeh MA, Nicholas SB, Norris KC, Vaziri ND. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant. 2013;28:2038-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Li S, Eguchi N, Lau H, Ichii H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | DeFronzo RA, Beckles AD. Glucose intolerance following chronic metabolic acidosis in man. Am J Physiol. 1979;236:E328-E334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Hayata H, Miyazaki H, Niisato N, Yokoyama N, Marunaka Y. Lowered extracellular pH is involved in the pathogenesis of skeletal muscle insulin resistance. Biochem Biophys Res Commun. 2014;445:170-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Franch HA, Raissi S, Wang X, Zheng B, Bailey JL, Price SR. Acidosis impairs insulin receptor substrate-1-associated phosphoinositide 3-kinase signaling in muscle cells: consequences on proteolysis. Am J Physiol Renal Physiol. 2004;287:F700-F706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Bellasi A, Di Micco L, Santoro D, Marzocco S, De Simone E, Cozzolino M, Di Lullo L, Guastaferro P, Di Iorio B; UBI study investigators. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016;17:158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Greco EA, Lenzi A, Migliaccio S. Role of Hypovitaminosis D in the Pathogenesis of Obesity-Induced Insulin Resistance. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Oh J, Riek AE, Darwech I, Funai K, Shao J, Chin K, Sierra OL, Carmeliet G, Ostlund RE Jr, Bernal-Mizrachi C. Deletion of macrophage Vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 2015;10:1872-1886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Lee S, Clark SA, Gill RK, Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134:1602-1610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Huang Y, Ishizuka T, Miura A, Kajita K, Ishizawa M, Kimura M, Yamamoto Y, Kawai Y, Morita H, Uno Y, Yasuda K. Effect of 1 alpha,25-dihydroxy vitamin D3 and vitamin E on insulin-induced glucose uptake in rat adipocytes. Diabetes Res Clin Pract. 2002;55:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Xu Z, Gong R, Luo G, Wang M, Li D, Chen Y, Shen X, Wei X, Feng N, Wang S. Association between vitamin D3 levels and insulin resistance: a large sample cross-sectional study. Sci Rep. 2022;12:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | He S, Yu S, Zhou Z, Wang C, Wu Y, Li W. Effect of vitamin D supplementation on fasting plasma glucose, insulin resistance and prevention of type 2 diabetes mellitus in non-diabetics: A systematic review and meta-analysis. Biomed Rep. 2018;8:475-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Niroomand M, Fotouhi A, Irannejad N, Hosseinpanah F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? Diabetes Res Clin Pract. 2019;148:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 27. | Wenclewska S, Szymczak-Pajor I, Drzewoski J, Bunk M, Śliwińska A. Vitamin D Supplementation Reduces Both Oxidative DNA Damage and Insulin Resistance in the Elderly with Metabolic Disorders. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23:804-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 599] [Cited by in F6Publishing: 782] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 29. | Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3103] [Cited by in F6Publishing: 2975] [Article Influence: 228.8] [Reference Citation Analysis (0)] |
| 30. | Lee DM, Knight-Gibson C, Samuelsson O, Attman PO, Wang CS, Alaupovic P. Lipoprotein particle abnormalities and the impaired lipolysis in renal insufficiency. Kidney Int. 2002;61:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Koppe L, Pelletier CC, Alix PM, Kalbacher E, Fouque D, Soulage CO, Guebre-Egziabher F. Insulin resistance in chronic kidney disease: new lessons from experimental models. Nephrol Dial Transplant. 2014;29:1666-1674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Kielstein JT, Zoccali C. Asymmetric dimethylarginine: a cardiovascular risk factor and a uremic toxin coming of age? Am J Kidney Dis. 2005;46:186-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Dzúrik R, Spustová V, Lajdová I. Inhibition of glucose utilization in isolated rat soleus muscle by pseudouridine: implications for renal failure. Nephron. 1993;65:108-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Lee W, Lee HJ, Jang HB, Kim HJ, Ban HJ, Kim KY, Nam MS, Choi JS, Lee KT, Cho SB, Park SI. Asymmetric dimethylarginine (ADMA) is identified as a potential biomarker of insulin resistance in skeletal muscle. Sci Rep. 2018;8:2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | D'Apolito M, Du X, Zong H, Catucci A, Maiuri L, Trivisano T, Pettoello-Mantovani M, Campanozzi A, Raia V, Pessin JE, Brownlee M, Giardino I. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest. 2010;120:203-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018;93:741-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 37. | Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y, Soula HA, Fouque D, Soulage CO. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013;24:88-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 38. | Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20:604-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 39. | Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Hosoya K, Minakuchi H, Wakino S, Fujimura K, Hasegawa K, Komatsu M, Yoshifuji A, Futatsugi K, Shinozuka K, Washida N, Kanda T, Tokuyama H, Hayashi K, Itoh H. Insulin resistance in chronic kidney disease is ameliorated by spironolactone in rats and humans. Kidney Int. 2015;87:749-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Preiss D, Zetterstrand S, McMurray JJ, Ostergren J, Michelson EL, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Gerstein HC, Sattar N; Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity Investigators. Predictors of development of diabetes in patients with chronic heart failure in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) program. Diabetes Care. 2009;32:915-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Finsen SH, Hansen MR, Hoffmann-Petersen J, Højgaard HF, Mortensen SP. Eight weeks of mineralocorticoid blockade does not improve insulin sensitivity in type 2 diabetes. Physiol Rep. 2021;9:e14971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 43. | Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15:4137-4142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 205] [Cited by in F6Publishing: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 44. | American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S125-S143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 431] [Article Influence: 215.5] [Reference Citation Analysis (0)] |
| 45. | Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15:569-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 348] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 46. | . Diagnostic ultrasound. Vet Clin North Am Equine Pract. 1986;2:1-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 47. | Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038-2049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 630] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 48. | Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 550] [Cited by in F6Publishing: 642] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 49. | Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54:2460-2470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 467] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 50. | Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1612] [Cited by in F6Publishing: 1511] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 51. | Abe M, Okada K, Kikuchi F, Matsumoto K. Clinical investigation of the effects of pioglitazone on the improvement of insulin resistance and blood pressure in type 2-diabetic patients undergoing hemodialysis. Clin Nephrol. 2008;70:220-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Abe M, Okada K, Maruyama T, Maruyama N, Soma M, Matsumoto K. Clinical effectiveness and safety evaluation of long-term pioglitazone treatment for erythropoietin responsiveness and insulin resistance in type 2 diabetic patients on hemodialysis. Expert Opin Pharmacother. 2010;11:1611-1620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005;172:213-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 704] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 54. | Mandarino LJ, Gerich JE. Prolonged sulfonylurea administration decreases insulin resistance and increases insulin secretion in non-insulin-dependent diabetes mellitus: evidence for improved insulin action at a postreceptor site in hepatic as well as extrahepatic tissues. Diabetes Care. 1984;7 Suppl 1:89-99. [PubMed] [Cited in This Article: ] |
| 55. | Müller G. The molecular mechanism of the insulin-mimetic/sensitizing activity of the antidiabetic sulfonylurea drug Amaryl. Mol Med. 2000;6:907-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Hanefeld M. Pioglitazone and sulfonylureas: effectively treating type 2 diabetes. Int J Clin Pract Suppl. 2007;61:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Bailey CJ. Insulin resistance: Impact on therapeutic developments in diabetes. Diab Vasc Dis Res. 2019;16:128-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Abe M, Okada K, Maruyama T, Maruyama N, Matsumoto K. Combination therapy with mitiglinide and voglibose improves glycemic control in type 2 diabetic patients on hemodialysis. Expert Opin Pharmacother. 2010;11:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | van de Laar FA. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc Health Risk Manag. 2008;4:1189-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 60. | Nakamura K, Yamagishi S, Matsui T, Inoue H. Acarbose, an alpha-glucosidase inhibitor, improves insulin resistance in fructose-fed rats. Drugs Exp Clin Res. 2005;31:155-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072-2077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1820] [Cited by in F6Publishing: 1609] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 62. | Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1103] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 63. | Bayer HealthCare Pharmaceuticals Inc. Product Information: PRECOSER (acarbose tablets). 2015. [cited 10 June 2022]. Available from: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=c85bc02a-bf18-4690-84b8-2c256bce5f9f. [Cited in This Article: ] |
| 64. | Lin WH, Yang CY, Kuo S, Kuo TH, Roan JN, Li CY, Wang MC, Ou HT. Hepatic and cardiovascular safety of acarbose among type 2 diabetes patients with end-stage renal disease: A nationwide population-based longitudinal study. Diabetes Res Clin Pract. 2021;172:108489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2:101-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1354] [Cited by in F6Publishing: 1331] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 67. | Vella A. Mechanism of action of DPP-4 inhibitors--new insights. J Clin Endocrinol Metab. 2012;97:2626-2628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK; CARMELINA Investigators. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019;321:69-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 679] [Cited by in F6Publishing: 729] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 69. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1831] [Cited by in F6Publishing: 1804] [Article Influence: 200.4] [Reference Citation Analysis (0)] |
| 70. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2548] [Cited by in F6Publishing: 2509] [Article Influence: 228.1] [Reference Citation Analysis (0)] |
| 71. | Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 72. | Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, Lasseter KC, Kipnes MS, Snyder K, Hilliard D, Tanen M, Cilissen C, De Smet M, de Lepeleire I, Van Dyck K, Wang AQ, Zeng W, Davies MJ, Tanaka W, Holst JJ, Deacon CF, Gottesdiener KM, Wagner JA. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612-4619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 73. | Azuma K, Rádiková Z, Mancino J, Toledo FG, Thomas E, Kangani C, Dalla Man C, Cobelli C, Holst JJ, Deacon CF, He Y, Ligueros-Saylan M, Serra D, Foley JE, Kelley DE. Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:459-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Yang L, Yuan J, Zhou Z. Emerging roles of dipeptidyl peptidase 4 inhibitors: anti-inflammatory and immunomodulatory effect and its application in diabetes mellitus. Can J Diabetes. 2014;38:473-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Trzaskalski NA, Fadzeyeva E, Mulvihill EE. Dipeptidyl Peptidase-4 at the Interface Between Inflammation and Metabolism. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420912972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 76. | Wu S, Gao L, Cipriani A, Huang Y, Yang Z, Yang J, Yu S, Zhang Y, Chai S, Zhang Z, Sun F, Zhan S. The effects of incretin-based therapies on β-cell function and insulin resistance in type 2 diabetes: A systematic review and network meta-analysis combining 360 trials. Diabetes Obes Metab. 2019;21:975-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Hattori S. Omarigliptin decreases inflammation and insulin resistance in a pleiotropic manner in patients with type 2 diabetes. Diabetol Metab Syndr. 2020;12:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Yaribeygi H, Sathyapalan T, Sahebkar A. Molecular mechanisms by which GLP-1 RA and DPP-4i induce insulin sensitivity. Life Sci. 2019;234:116776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 79. | Nuffer WA, Trujillo JM. Liraglutide: A New Option for the Treatment of Obesity. Pharmacotherapy. 2015;35:926-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 80. | Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, Vergès B, Marre M. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46:100-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 81. | Bednarz K, Kowalczyk K, Cwynar M, Czapla D, Czarkowski W, Kmita D, Nowak A, Madej P. The Role of Glp-1 Receptor Agonists in Insulin Resistance with Concomitant Obesity Treatment in Polycystic Ovary Syndrome. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 82. | Górriz JL, Soler MJ, Navarro-González JF, García-Carro C, Puchades MJ, D'Marco L, Martínez Castelao A, Fernández-Fernández B, Ortiz A, Górriz-Zambrano C, Navarro-Pérez J, Gorgojo-Martinez JJ. GLP-1 Receptor Agonists and Diabetic Kidney Disease: A Call of Attention to Nephrologists. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 83. | Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2303] [Cited by in F6Publishing: 2391] [Article Influence: 298.9] [Reference Citation Analysis (0)] |
| 84. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2826] [Cited by in F6Publishing: 3531] [Article Influence: 706.2] [Reference Citation Analysis (0)] |
| 85. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1586] [Cited by in F6Publishing: 2557] [Article Influence: 639.3] [Reference Citation Analysis (1)] |
| 86. | Obata A, Kubota N, Kubota T, Iwamoto M, Sato H, Sakurai Y, Takamoto I, Katsuyama H, Suzuki Y, Fukazawa M, Ikeda S, Iwayama K, Tokuyama K, Ueki K, Kadowaki T. Tofogliflozin Improves Insulin Resistance in Skeletal Muscle and Accelerates Lipolysis in Adipose Tissue in Male Mice. Endocrinology. 2016;157:1029-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 87. | Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, Mayoux E, Kaneko S, Ota T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine. 2017;20:137-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 88. | Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 606] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 89. | Okada K, Takahashi Y, Okawa E, Onishi Y, Hagi C, Aoki K, Shibahara H, Higuchi T, Nagura Y, Kanmatsuse K, Takahashi S. Relationship between insulin resistance and uremic toxins in the gastrointestinal tract. Nephron. 2001;88:384-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Grassi G, Seravalle G, Dell'Oro R, Trevano FQ, Bombelli M, Scopelliti F, Facchini A, Mancia G; CROSS Study. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens. 2003;21:1761-1769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 91. | Jin HM, Pan Y. Angiotensin type-1 receptor blockade with losartan increases insulin sensitivity and improves glucose homeostasis in subjects with type 2 diabetes and nephropathy. Nephrol Dial Transplant. 2007;22:1943-1949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | DREAM Trial Investigators, Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 476] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 93. | NAVIGATOR Study Group; McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477-1490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 432] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 94. | Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383:2219-2229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 1173] [Article Influence: 293.3] [Reference Citation Analysis (1)] |
| 95. | Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD Investigators. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021;385:2252-2263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 632] [Article Influence: 210.7] [Reference Citation Analysis (0)] |
| 96. | Marzolla V, Feraco A, Limana F, Kolkhof P, Armani A, Caprio M. Class-specific responses of brown adipose tissue to steroidal and nonsteroidal mineralocorticoid receptor antagonists. J Endocrinol Invest. 2022;45:215-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | Jenkins HN, Rivera-Gonzalez O, Gibert Y, Speed JS. Endothelin-1 in the pathophysiology of obesity and insulin resistance. Obes Rev. 2020;21:e13086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 98. | Rivera-Gonzalez O, Wilson NA, Coats LE, Taylor EB, Speed JS. Endothelin receptor antagonism improves glucose handling, dyslipidemia, and adipose tissue inflammation in obese mice. Clin Sci (Lond). 2021;135:1773-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Melnick JZ, Miller MG, Pergola PE, Perkovic V, Tobe S, Yi T, Wigderson M, de Zeeuw D; SONAR Committees and Investigators. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937-1947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 396] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 100. | Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7:216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 186] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 101. | Mir R, Elfaki I, Duhier FMA, Alotaibi MA, AlAlawy AI, Barnawi J, Babakr AT, Mir MM, Mirghani H, Hamadi A, Dabla PK. Molecular Determination of mirRNA-126 rs4636297, Phosphoinositide-3-Kinase Regulatory Subunit 1-Gene Variability rs7713645, rs706713 (Tyr73Tyr), rs3730089 (Met326Ile) and Their Association with Susceptibility to T2D. J Pers Med. 2021;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Lin B, Li Z, Park K, Deng L, Pai A, Zhong L, Pirrung MC, Webster NJ. Identification of novel orally available small molecule insulin mimetics. J Pharmacol Exp Ther. 2007;323:579-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Lan ZJ, Lei Z, Yiannikouris A, Yerramreddy TR, Li X, Kincaid H, Eastridge K, Gadberry H, Power C, Xiao R, Lei L, Seale O, Dawson K, Power R. Non-peptidyl small molecule, adenosine, 5'-Se-methyl-5'-seleno-, 2',3'-diacetate, activates insulin receptor and attenuates hyperglycemia in type 2 diabetic Lepr(db/db) mice. Cell Mol Life Sci. 2020;77:1623-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |