Copyright
©The Author(s) 2023.
World J Diabetes. Mar 15, 2023; 14(3): 222-233
Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.222
Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.222
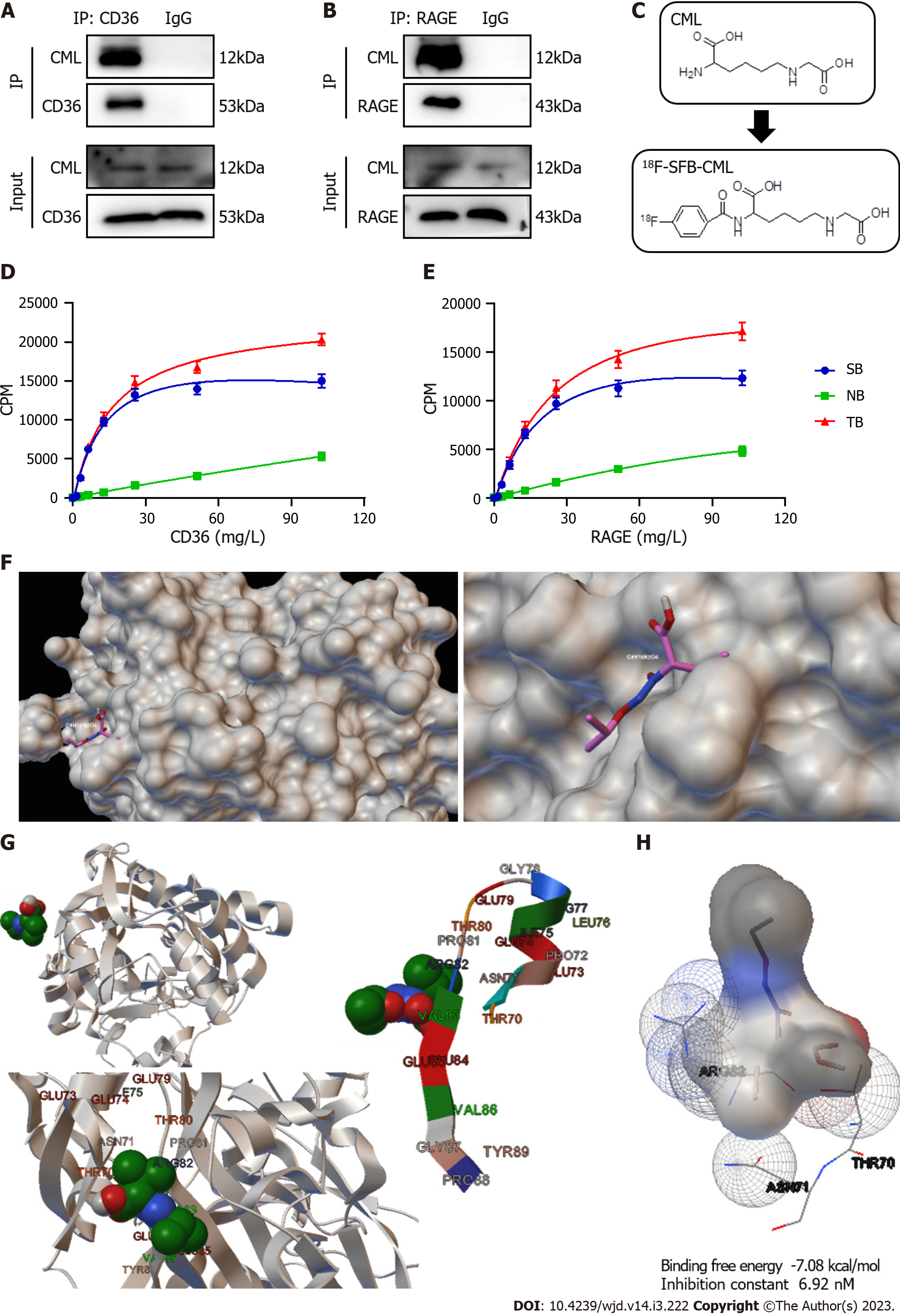
Figure 2 Nε-(carboxymethyl)lysine has a higher binding affinity to cluster of differentiation 36 than to receptor for advanced glycation end products.
A and B: Detection of Nε-(carboxymethyl)lysine (CML) binding to cluster of differentiation 36 (CD36) (A) and to receptor for advanced glycation end products (RAGE) (B) by immunoprecipitation; C: Synthesis of N-succinimidyl-4-18F-fluorobenzoate-CML; D and E: Detection of the specific binding between CML and CD36 (D) and between CML and RAGE (E) with radioreceptor ligand binding assays; F: Molecular docking model of CML-CD36 binding; G: Detail of the binding site of CML to CD36; H: Amino acids for the binding interaction between CML and CD36. SB: Specific binding; NB: Non-specific binding; TB: Total binding. CPM: Counts/minute.
- Citation: Wang ZQ, Yao HP, Sun Z. Nε-(carboxymethyl)lysine promotes lipid uptake of macrophage via cluster of differentiation 36 and receptor for advanced glycation end products. World J Diabetes 2023; 14(3): 222-233
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/222.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.222