Copyright
©The Author(s) 2023.
World J Diabetes. Feb 15, 2023; 14(2): 76-91
Published online Feb 15, 2023. doi: 10.4239/wjd.v14.i2.76
Published online Feb 15, 2023. doi: 10.4239/wjd.v14.i2.76
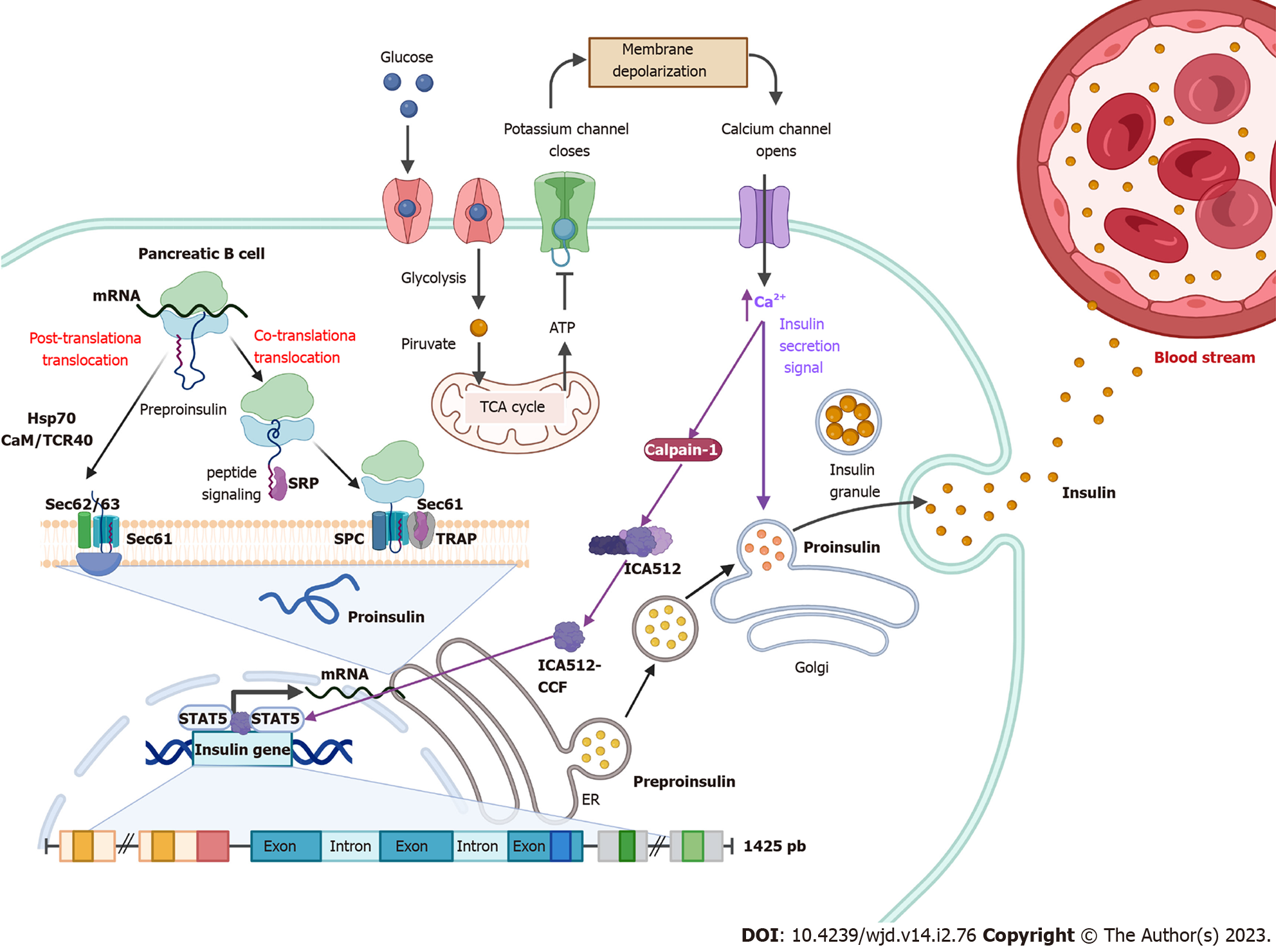
Figure 1 Insulin synthesis.
Following food intake, glucose is internalized into pancreatic β cells and its degradation through glycolysis and the tricarboxylic acid cycle is initiated. Intracellular ATP levels increase, which generates the closure of K+ channels, causing a change in membrane permeability opening Ca2+ channels. Elevated Ca2+ intracellular levels activate calpain-1, a protease that cleaves a cytosolic fragment of islet cell autoantigen 512. The free fragment islet cell autoantigen 512 targets the nucleus and binds to signal transducer and activator of transcription 5, which in turn promotes increased transcription of the insulin gene to mRNA. In the cytosol, insulin is translated as pre-proinsulin that includes a nuclear transport signal peptide that guides pre-proinsulin to the rough endoplasmic reticulum (ER) membrane for translocation to the ER cisternae via two mechanisms: a signal recognition particle-dependent cotranslational translocation; and a signal recognition particle-independent post-translational translocation mechanism. Pro-insulin is generated and folds and stabilizes in its three-dimensional configuration. After acquiring three-dimensional folding, pro-insulin is transferred from the ER to the Golgi via vesicles where pro-insulin is converted to insulin. Also, elevated Ca2+ intracellular levels induce the remodeling of the cytoskeleton and the translocation of insulin granules to the plasma membrane to be subsequently secreted to the blood stream.
- Citation: De la Cruz-Concepción B, Flores-Cortez YA, Barragán-Bonilla MI, Mendoza-Bello JM, Espinoza-Rojo M. Insulin: A connection between pancreatic β cells and the hypothalamus. World J Diabetes 2023; 14(2): 76-91
- URL: https://www.wjgnet.com/1948-9358/full/v14/i2/76.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i2.76