Published online Apr 15, 2022. doi: 10.4239/wjd.v13.i4.308
Peer-review started: May 9, 2021
First decision: July 3, 2021
Revised: August 9, 2021
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: April 15, 2022
Diabetic kidney disease (DKD) is one of the major chronic complications of diabetes mellitus (DM), as well as a main cause of end-stage renal disease. Over the last few years, substantial research studies have revealed a contributory role of gut microbiota in the process of DM and DKD. Metabolites of gut microbiota like lipopolysaccharide, short-chain fatty acids, and trimethylamine N-oxide are key mediators of microbial–host crosstalk. However, the underlying mechanisms of how gut microbiota influences the onset and progression of DKD are relatively unknown. Besides, strategies to remodel the composition of gut microbiota or to reduce the metabolites of microbiota have been found recently, representing a new potential remedial target for DKD. In this mini-review, we will address the possible contribution of the gut microbiota in the pathogenesis of DKD and its role as a therapeutic target.
Core Tip: This minireview consolidates the potential role of gut microbiota in the pathogenesis and as a therapeutic target of diabetic kidney disease. It is known that metabolites of gut microbiota such as trimethylamine N-oxide, short-chain fatty acids, and lipopolysaccharides are important mediators of microbial-host crosstalk. However, the main mechanism of how the gut microbiota specifically affects the occurrence and progress of diabetic kidney disease has not yet been fully explored.
- Citation: Lin JR, Wang ZT, Sun JJ, Yang YY, Li XX, Wang XR, Shi Y, Zhu YY, Wang RT, Wang MN, Xie FY, Wei P, Liao ZH. Gut microbiota and diabetic kidney diseases: Pathogenesis and therapeutic perspectives. World J Diabetes 2022; 13(4): 308-318
- URL: https://www.wjgnet.com/1948-9358/full/v13/i4/308.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i4.308
Diabetes mellitus (DM) continues to be one of the most challenging and economically costly diseases in the world, with its prevalence and incidence increasing[1]. About 20%-40% of the affected population will develop into diabetic kidney disease (DKD)[2], which is the primary contributor of end-stage renal disease (ESRD). The global incidence rate of diabetes in 2019 was expected to be 9.3% (463 million people) and may rise to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045[3-5]. The direct health expenses worldwide on diabetes in 2019 were predicted to be USD 760 billion and are projected to increase to 825 billion dollars by 2030 and 845 billion dollars by 2045[6]. The concern is that the prevalence of diabetes might continue to build up due to significantly expanded incidence of childhood obesity.
DKD can occur in type 1 diabetes, type 2 diabetes, and other secondary diabetes. The development of moderately increased albuminuria in patients with type 1 diabetes typically occurs 5 to 15 years after diabetes initiation and progresses through time[7,8]. In a systematic review encompassing nine longitudinal studies of 7938 patients with type 1 diabetes and moderately increased albuminuria, the total incidence rate of moderately increased albuminuria was 28% over the mean 15-year duration of diabetes[9]. In comparison to patients with normoalbuminuria, the relative risk for all-cause mortality was 1.8 (95% confidence interval: 1.5-2.1), with a suggestion of a similar relative risk for cardiovascular mortality[9,10]. Among nearly 5100 patients with type 2 diabetes included in the United Kingdom prospective diabetes study[11], regarding the occurrence and progression of nephropathy, the results are reported as follows: Ten years after the diagnosis of diabetes, the percentages of cases with moderately elevated, those with severely elevated urine albumin, and those with plasma creatinine concentrations elevated to > 175 μmol/L (2.0 mg/dL) or requiring kidney substitution treatment were 25%, 5%, and 0.8%, respectively.
The human gut nurtures more than 100 trillion microbial cells. The functional gut microbiota serves particular roles in many metabolic aspects of the host, including nutritional metabolism, alloantigen and medicinal metabolism, maintaining the integrity of the intestinal mucosal barricade structure, immune regulation, as well as resistance to pathogens[12]. Microbial cells are susceptibility factors for the development of nephropathy in individuals with a predisposition to nephropathy, such as patients with DM[13,14]. The intestinal flora may influence the development and progression process of DKD by modifying the endocrine functions of the gut and the components of microbial metabolism products, and vice versa. Besides, hyperglycemia and progressive kidney disease determine alterations of the gut microbiota[15,16].
In this article, we review the quantitative and qualitative changes in the gut microbiota of DKD patients that lead to this symbiotic disorder and how it contributes to the progression of DKD, and review well-targeted interferences that can reconstruct the symbiotic relationship.
DKD is a complicated and miscellaneous disease with numerous interrelated etiologic pathways. Patients with DKD have four main glomerular histopathological changes: Mesangial expansion, glomerular basement membrane (GBM) thickening, podocyte effacement, and glomerular sclerosis. It was believed that these histopathological changes were mainly due to the metabolic and hemodynamic disorders found in diabetes. Hemodynamic derangements are defined as the hyperfiltration which is due to vasoconstriction of efferent arterioles following the activation of renin-angiotensin-aldosterone system (RAAS) under the stimulation of hyperglycemia. Nevertheless, in recent years it has become more and more apparent that despite the irrefutable central role of hyperglycemia in the development of DKD, it is not the only contributor to DKD. In general, the development of DKD involves several pathophysiological pathways including hemodynamic pathways, metabolic pathways, inflammatory pathways, and autophagy pathways.
The changes in renal hemodynamics are partially regulated by vasoactive hormones, especially angiotensin II (Ang II) and endothelin (ET). In cultured rat mesangial cells, glucose increases Ang II production in a concentration-dependent manner, which results in stimulation of transforming growth factor-β1 (TGF-β1) secretion, decreased matrix degradation, and increased matrix accumulation[17]. Temporarily blocking the prediabetic rats’ renin–angiotensin system for 7 wk resulted in a sustained reduction in collagen accumulation and gene expression of connective tissue growth factor (CTGF), which mediates downstream events of TGF-β and stimulates fibroblast proliferation and extracellular matrix (ECM) protein synthesis[18,19]. In response to various factors, mesangial cells can release ET-1 and ET receptors, activation of which leads to a complex signaling cascade with resultant stimulation of mesangial cell hypertrophy, proliferation, contraction, and ECM accumulations[20].
The metabolic pathways including four different entities: The polyol pathway, hexosamine pathway, production of advanced glycation end products (AGEs), and activation of protein kinase C (PKC)[21]. Aldose reductase is the first enzyme in the polyol pathway. Studies have shown that the hemodynamic changes caused by early diabetes and the increase in vascular albumin infiltration and urinary albumin excretion (UAE) are phenomena associated with aldose reductase[22]. The hexosamine pathway originates in the third phase of glycolysis, where fructose-6-phosphate is transformed into glucosamine-6-phosphate. Glucosamine-6-phosphate later is utilized as a substrate which augments the transcription of the inflammatory cytokines tumor necrosis factor-α (TNF-α) and TGF-β1[23], which we will discuss in the inflammatory pathways later. Tissue protein glycosylation is also one of the causes of diabetic nephropathy and other microvascular complications. In a long-term hyperglycemia state, part of the excess glucose will bind to free amino acids in the circulation or tissue proteins. The non-enzymatic reaction initially forms reversible early glycosylation products, and then forms irreversible AGEs. Long-term infusion of AGE-albumin to non-diabetic animals led to glomerular enlargement, GBM hyperplasia, mesangial ECM swelling, and albuminuria, which are all consistent with the glomerulopathy analogous to DKD[24]. Hyperglycemia-induced PKC activation in cultured mesangial cells or diabetic glomeruli is associated with a number of aberrations, namely, elevated arachidonic acid secretion and prostaglandins synthesis, elevated expression of fibronectin, α1(IV) collagen, and TGF-β1, and depressed Na+K+-ATPase action[25].
Various growth factors and cytokines may affect renal function directly or indirectly and perform their actions by stimulating other factors. As mentioned before, in cultured mesangial cells, high glucose or Ang-stimulated production of matrix proteins is partially regulated by TGF-β. The mechanisms involve suppression of matrix metalloproteinase synthesis, incentive of metalloproteinase inhibitor production, enhanced CTGF expression, etc.[19,26]. The expression of vascular endothelial growth factor (VEGF) is pronounced in quite few cells including glomerular visceral epithelial cells and tubular epithelial cells, where VEGF is able to induce a proliferative and an antiapoptotic response[27]. The direct evidence that VEGF is a mediator of DKD was collected from research, in which the weight of the kidney, the glomerular volume, the thickness of basement membrane rose while UAE descended in VEGF antibody-treated db/db mice. VEGF antibody administration tended to reduce expansion in total mesangial volume[28]. Each cytokine has several different effects. IL-1 takes a part in the progression of intra-glomerular hemodynamic aberrations associated with prostaglandin production by mesangial cells and can directly increase vascular endothelial cell permeability[29,30]. The expression of renal IL-6 positively correlates with mesangial hyperplasia and tubular atrophy in various kidney disease models[31]. IL-18 triggers the secretion of interferon gamma and results in producing additional inflammatory cytokines including IL-1 and TNF, over-expression of adhesion molecules, as well as inducing endothelial cell apoptosis[32]. TNF is recognized to play a crucial part in the pathogenesis of DKD. TNF is not only cytotoxic to kidney cells, which can induce direct kidney damage, but also involved in processes such as the induction of apoptosis and necrotic cell death[33]. Studies have shown that TNF plays an important part in the progression of kidney hypertrophy and hypofunction, which are the two major changes in the preliminary stages of DKD, indicating that renal level of TNF may even have the potential to be used as a marker for early stage of DKD[34].
Autophagy (originating from the Greek word meaning "self-eating") is a basic cellular process sending intracellular components to lysosomes to be degraded in order to sustain homeostasis and cellular integrality[35]. Podocytes had a high basal level of autophagy. However, diabetic condition in vivo and high glucose conditions in vitro impaired autophagy, resulting in lysosome dysfunction and apoptosis, as well as autophagy defects leading to podocyte damage[36]. Because the dynamics of the endoplasmic reticulum (ER) appeared to have a crucial function in modulating autophagic fluxes, the cytoprotective capacity of the ER might fail under high glucose-induced unrelieved stress, which causes autophagy disruption, speeding up the deterioration of DKD[37].
The components and activeness of the intestinal flora are symbiotic with the host since birth and are contingent on complex interactions which depend on the host genome, nutrition, and lifestyle. The gut microbiota plays an important role in maintaining the gut in normal individuals and human health as a whole, and its dysfunction is tightly correlated with the occurrence of DM and the progression to DKD. Metabolome-based genome-wide association studies showed that patients with T2DM are distinguished by moderate dysbiosis of the intestinal microflora, for example, by decreased abundance of some prevalent butyrate-producing bacteria, including Clostridium difficile SS3/4, Escherichia coli, Prevotella, Roscoidium intestinalis, and Roscoidium chrysogenum, as well as by an elevated number of diverse potential pathogens, including Bacteroides caccae, Clostridium hathewayi, Clostridium ramosum, Clostridium symbiosum, Eggerthella lenta, and Escherichia coli, on top of which, there is an enrichment of the identified mucin-degrading species Akkermansia muciniphila and sulphate-reducing species Desulfovibrio sp. 3_1_syn3[38]. Several pieces of high quality data from the United States Human Microbiome Project (HMP)[39], European Metagenomics of the Human Intestinal Tract (Meta HIT)[40], and several other studies have proven the favorable effects of the balanced intestinal flora on health all the way to the genetic layer, while Tao et al[41] revealed that the abundance of the intestinal microflora and the degree of diversity of bacterial groups were significantly different in DM with respect to healthy controls, and DKD with respect to DM. Interestingly, the variables of g_Prevotella_9 (AUC = 0.9) allowed precise identification of DM from age- and sex-matched healthy controls, and the variables of g_Escherichia-Shigella and g_Prevotella_9 (AUC = 0.86) allowed precise identification of DKD from age- and sex-matched DM patients[41].
The gut microbiota participates in the regulation of various host metabolic pathways. Disorders of the gut environment and associated variations in the makeup of the gut microflora, as well as the metabolites produced, represent a condition referred to as “intestinal dysbiosis”[42,43], leading to disorders of interactive host-microbiota metabolism, signal transduction, and immune-inflammatory axes, influence the gut, liver, kidney, muscle, and brain through physiological connection, and thus may trigger a systemic inflammatory response. Under normal circumstances, the gut barricade precludes the transfer of substances and microorganisms from the intracavity to the bloodstream; the gut barricade is composed of distinct constructions/systems: Tight junctions, intestinal epithelial cell membranes, mucus secretion, and immune defensive mechanisms of the gut lining[42,44]. However, intestinal dysbiosis may result in a “leaky gut syndrome”, with increased permeability that enables the leakage of pro-inflammatory bacterial products [e.g., lipopolysaccharide (LPS)], contributing to insulin resistance[45] as well as expediting the development of renal disorders in people with diabetes[14]. Microbial metabolites are essential intermediaries of microbial host crosstalk, engaging in the regulation of host metabolism and gut integrity.
Endotoxin, a phospholipid, is the hydrophobic anchor of LPS which comprises the external layer of the majority of Gram-negative bacteria. Salguero et al[46] revealed a significant relevance between the dysbiosis of Gram-negative bacteria which includes increasing relative abundance of Proteobacteria, Verrucomicrobia, and Fusobacteria, raised LPS concentrations, and accumulated state of inflammatory biomarkers consisting of C-reactive protein (CRP), TNF-α, and IL-6 in DKD patients in contrast to the controls[46]. Also, as a result of the leaky gut syndrome, LPS translocation which leads to high circulating levels of LPS, a condition known as “endotoxemia”, stimulates immune system cells, especially macrophages and endothelial cells. In macrophages, LPS activates IL-1R-associated kinase (IRAK) through TLR4-mediated MyD88 and MD2 signaling, with ensuing induction of TNF receptor-associated factor 6 (TRAF6) binding with IRAK and other proteins forming a large complex, catalyzing the synthesis of a Lys 63-linked polyubiquitin chain of TRAF6 and finally resulting in the activated transcription factor NF-κB and discharged pro-inflammatory cytokines[47], which is known to be important in the pathogenesis of DKD[48].
The hallmark feature of gut dysbiosis is a decrease in the levels of short chain fatty acids (SCFAs)-producing saccharolytic microbes. SCFAs are the end products of fermentation of dietary polysaccharides by intestinal microbiota, including acetate, propionate, butyrate, pentanoic acid, and isobutyric acid[49]. The functions of SCFAs are generally concerned with the activation of transmembrane G protein-coupled receptors (GPR) and the repression of histone acetylation (HDAC)[50], and the increase of glucagon-like peptide-1 (GLP-1) and GLP-2 production through GPR stimulation, along with elevated insulin expression and ensuing augmented insulin sensitivity and proliferation of pancreatic cells. Intriguingly, glucose homeostasis and feelings of satiety are both regulated by gut microbiota components like Bifidobacterium and Lactobacillus that enhance GLP-1 secretion[51]. Besides, SCFAs can inhibit oxidative stress and inflammation of glomerular mesangial cells induced by high glucose and LPS[52], as well as improve intestinal barrier function[53]. Sodium butyrate treatment markedly reduced the levels of glucose, creatinine, and urea in plasma, attenuated histological changes, involving fibrosis and collagen deposition, and curbed the activity of HDACs, eNOS, iNOS, fibronectin, TGF-β1, NF-κB, apoptosis, and DNA damage in diabetic kidneys[54]. However, not all the remedies of SCFAs showed favorable effects. Lu et al[55] discovered aberrant intestinal flora, elevated plasma acetate levels, raised proteinuria, thickened GBM, and loss of renal podocyte foot process in DM rats compared to control rats[55]. In addition, the amount of angiotensin II, angiotensin-converting enzyme, and angiotensin II type1 receptor boosted in DM rats’ kidneys, suggesting that redundant acetic acid produced from gut flora disorders may cause kidney damage by activating RAAS in the kidney. It is hypothesized that these differences of SCFAs studies may result from disparate animal models in disparate diseases as well as from the group, concentration, and timing of application of SCFAs.
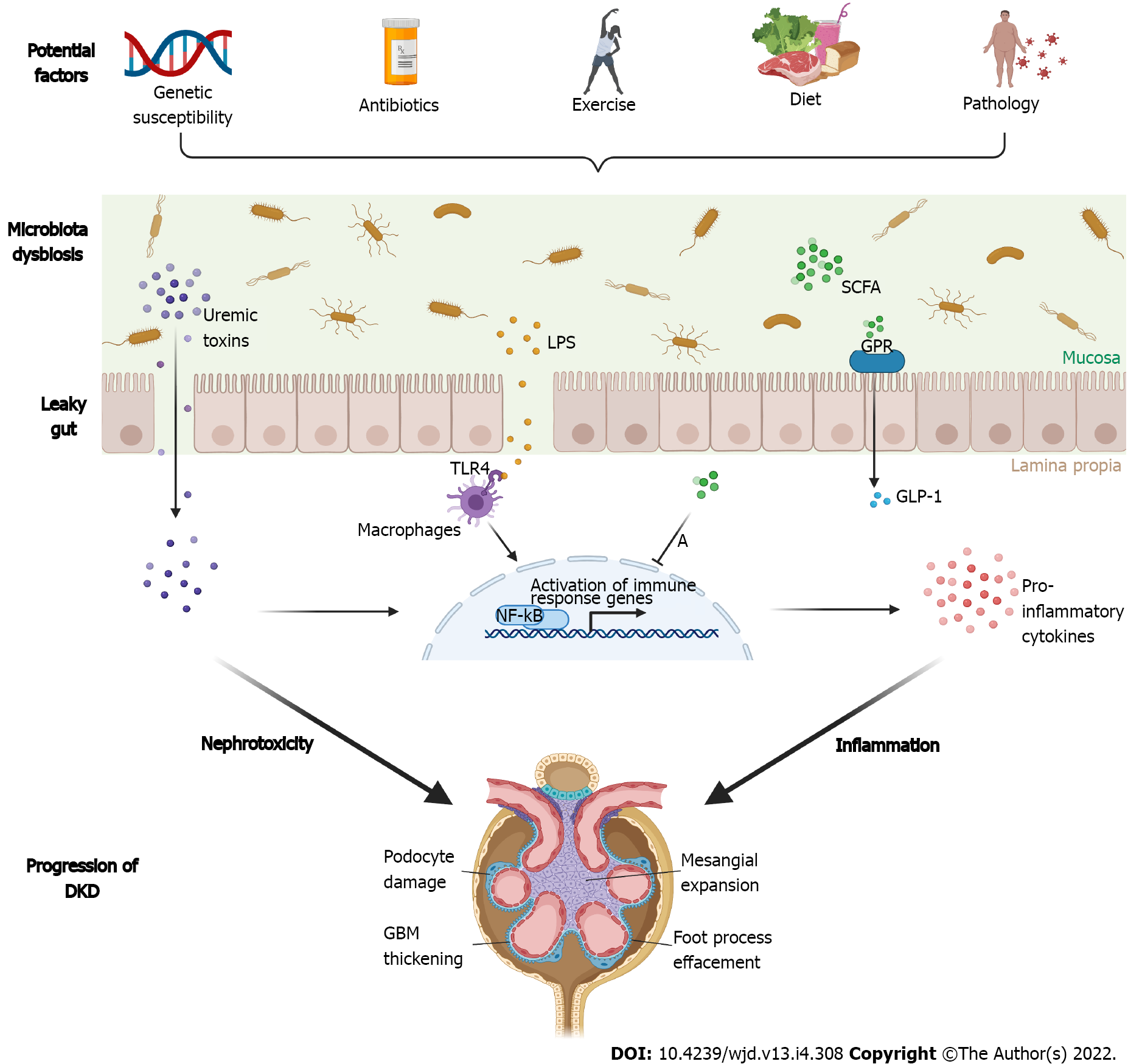
Imbalance of the gut microbiota is also a potential source of uremic toxins. Urea is derived in the liver from the urea cycle and its origin is dietary/endogenous amino acids and their decomposition in the peripheral tissues. The intestinal microbiota uses urease to convert urea into ammonia (NH3) and carbon dioxide. A portion of the ammonia goes through the urea cycle in the liver and is transformed back into urea, whereas the rest of the ammonia is transformed into ammonium hydroxide (NH4OH) and then excreted from the body with feces[13]. Changes in lifestyle and diet as well as reduced fiber consumption can cause imbalance in the intestinal flora and production of an overload of the uremic toxins [e.g., indoxyl sulfate (IS), phenyl sulfate (PS), p-cresyl sulfate (PCS), and trimethylamine-N-oxide (TMAO)]. Normally, the amount of IS receptors [aryl hydrocarbon receptors (AhRs)] may modulate podocyte functionality. Nevertheless, under conditions of imbalanced intestinal flora, AhRs are prolonged activated by broad exposure to IS, which results in progressive damage of podocytes and glomeruli including altered cell morphology, elevated levels of expression of pro-inflammatory cytokines and chemokines, declined podocyte differentiation, and reduced expression of cytoskeletal proteins[56]. Also, AhR was demonstrated to be able to interact with various signaling molecules such as NF-κB, which is responsible for the upregulation of proinflammatory proteins in uremic conditions[57]. Kikuchi et al[58] found that the amount of PS (an intestinal microflora-derived metabolite) increased with advancing diabetes in rats in which the human uremic toxin transporter SLCO4C1 was over-expressed in the kidney, whereas it declined in rats that showed limited proteinuria. In pilot models of DM, the giving of PS triggers albuminuria and podocyte injury. In a cohort of DM patients, PS levels were closely related to the baseline and forecasted advancement of albuminuria in patients with microalbuminuria over 2 years[58]. Figure 1 illustrates the pathogenic associations between gut dysbiotic microbiota and development of DKDs from the gut-kidney axis.
Exercise is considered to be an important potential factor in modification of gut microbiota, which could have both beneficial and harmful effects under some specific circumstances. Moderate levels of exercise may be able to keep balance of gut microbiota and reduce harmful bacteria in the digestive tract to some extent[59]. However, Cani et al[60] found that excessively intensive exercise may lead to increased permeability in the digestive tract[60].
Obviously, the host genome is the main risk determinant for a number of different diseases. Nonetheless, not alike the host genome, the genome of microorganisms in the host can be changed. Through the administration of prebiotics (dietary foods that boost the growth or performance of particular microorganisms), probiotics (live bacteria), synbiotics (mixtures of probiotics and prebiotics), as well as antibiotics, people are able to alter the composition of the intestinal microbiota themselves and thereby modify the resultant metabolites.
Animal studies found that high-fat-fed diabetic mice treated with prebiotics (fructo-oligosaccharides, FOS) not only had higher levels of intestinal Bifidobacterial and colonic GLP-1 precursor and reduced endotoxaemia, but also obtained improvement on their glucose tolerance and insulin resistance[61]. This dietary shift method also worked in germ-free mice colonized with a synthetic community where at day 35 (7 d following the change to the FOS diet), there was a distinct decline in Bacteroides caccae enrichment and a concurrent increment in B. caccae enrichment. Importantly, during the same period, there was a significant reduction in the level of IS in the host, and this decrease remained unchanged after 1 wk, suggesting a steady drop in the production of uremic toxins[62]. Li et al[63] reported that when feeding diabetic rats with a high-fiber diet, and feeding diabetic control rats with a normal diet or a zero-fiber diet, the former was less likely to fall into the DKD phase featured with albuminuria, glomerular hypertrophy, podocyte injury, and interstitial fibrosis. Fiber can profitably reshape intestinal microbial ecosystem and improve microecology dysbiosis. For example, fiber allowed growth in density of fecal and systemic SCFAs through stimulating the colonization of SCFA-producing bacteria such as the genera Prevotella and Bifidobacterium. Besides, fiber may intervene the progression of DKD by diminishing the expression of genes which are responsible for the generation of inflammatory cytokines, chemokines, and fibrosis-promoting proteins. SCFAs were nephroprotective in diabetic mice, providing that GPR43 or GPR109A is present. In vitro cellular experiments revealed that SCFAs could regulate hyperglycemia-induced inflammation in renal tubular cells and podocytes[63].
Bohlouli et al[64] analyzed data from 340 DKD patients by systematically reviewing and quantitatively synthesizing seven RCTs. They found that probiotics consumption beneficially impact the inflammation and oxidative stress biomarkers by significantly reducing high-sensitivity C-reactive protein (hs-CRP) and malondialdehyde (MDA) plus increasing glutathione (GSH) and total antioxidant capacity (TAC) in subjects. Yet, probiotics had no remarkable effect on concentrations of nitric oxide (NO). Subgroup analysis indicated that when the probiotic dosage was greater 5 billion CFU per day, the total impact of probiotics on serum TAC concentrations was more prominent[64]. Vlachou et al[65] concluded that most studies showed the beneficial effects of supplementary probiotics in decreasing inflammation and oxidative stress and improving biomarkers of kidney function in DKD patients, and the majority of microbes applied in the studies were in the genera of Lactobacillus and Bifidobacterium. Doses varied from 2 × 107 to 6 × 1010 CFU/g. The format of the probiotics differed among projects (capsules, pouches, soy milk, yogurt, and honey)[65]. Probiotics use may also help to reinforce the barrier function, through prevention of dysbiosis and regulation of cytoskeletal and tight junctional protein phosphorylation. Guo et al[66] illustrated that Bifidobacterium infantis and Lactobacillus acidophilus were able to safeguard the gut barrier from irritation by IL-1β and thereby preserve the intestinal permeability to an extent. The mechanism may be that the levels of occluding and claudin-1 were normalized and that the IL-1β-induced NF-κB activation was inhibited in Caco-2 cells[66]. Resta-Lenert and Barrett[67] remarked that when the epithelial cell lines were under the exposure to enteroinvasive Escherichia coli (EIEC), the application of S. thermophilus and L acidophilus could sustain and sometimes even strengthen the structures of cytoskeleton and tight junction proteins[67].
There are relatively few studies on synbiotics in DKD. In a randomized, double-blind and placebo-controlled trial encompassing 81 DM patients, the consumption of synbiotic bread containing Lactobacillus sporogenes and inulin caused a marked increment in levels of NO in the blood plasma and a remarkable drop in MDA concentrations compared to the probiotic and control breads. However, probiotic bread intake had no significant influence on the levels of TAC, GSH, and catalase in plasma, liver enzymes, calcium, iron, and magnesium in serum, and blood pressure in contrast to probiotic and control breads[68]. There is another study where patients with ESRD who were undergoing haemodialysis (HD) received synbiotic (Lactobacillus casei strain Shirota and Bifidobacterium breve strain Yakult as probiotics and galacto-oligosaccharides as prebiotics) for 2 wk. The results of the study demonstrated that p-cresol is a constipation-related uraemic toxin, and the three subjects with the highest serum p-cresol level were diabetic HD patients. The synbiotic regimen regularized defecation habits and reduced serum level of p-cresol in HD patients[69].
Hu et al[70] found that depletion of gut microbiota by antibiotics significantly alleviated tubulointerstitial injury, reduced IL-6 concentrations in the blood, and efficiently relieved glycemia in DM rats. Meanwhile, it rescued the increased urine albumin/creatinine ratio and N-acetyl-β-D-glucosidase /creatinine ratio. Intriguingly, in DM rats treated with antibiotics, the levels of acetate in the serum also declined significantly and were positively correlated with kidney cholesterol concentrations[70]. Similar results were found in diabetic rats by using broad-spectrum antibiotics, where not only the majority of the intestinal microbiota was thoroughly killed, but also the concentrations of acetate in plasma were reduced, intrarenal RAAS activation was effectively suppressed, and renal injury was mitigated[55]. Antibiotic therapy is unable to eliminate every microorganism that exists in the intestine of mice; however, it is possible to maintain the microbiome in quite low levels, which is why antibiotic therapy is commonly applied to acquire pseudo-germ-free mice in gut microbiota studies even if the requirement of germ-free mouse maintenance is rigid and difficult to fulfil in most laboratories. Nevertheless, a large amount of antibiotics may damage the kidneys of mice. Moreover, the use of antibiotics alone is not the best solution, because of the possible consequences of microbiome abatement, for example, antibiotic-related pathogen aggression[71]. The sensible application of antibiotics to achieve or enforce selection of strains colonized with specific metabolic traits is likely to present a plan that can achieve shrinkage of toxin production and preservation of many of the microbiota’s health benefits.
A more sustained and potent treatment to reconstruct a robust microbiome structure and functionality might include contiguous fecal microbiota transplantation (FMT) originating in healthy donors. Barba et al[72] found that FMT from healthy mice improved PCS accumulation, glucose tolerance, and albuminuria[72]. Reconstructing a "healthy microbiota" in patients shows great promise for rebuilding gut, immune, and metabolic homeostasis and it has been tested to be secure and well-tolerated in previous clinical trials[73,74].
Gut microbiota serves as a central part as the regulator in metabolic and inflammatory homeostasis, functioning as a link between the host and environmental influences. Constituent of the intestinal microbiota in DKD patients varies from that of the healthy population. Both animal and human studies have confirmed the correlation of gut dysregulation with DKD and associated metabolic disorders. Several studies have shown budding therapeutics against gut microbiota on glucose tolerance, insulin resistance, gut barrier integrity, endotoxaemia, uremic toxin, SCFA, TAC, and so forth, which may breed new methods for the prevention and treatment of DKD and relevant metabolic diseases. Howbeit, which gut microbiota constituents are the causes of renal injury and aberrant glucose metabolism, and which are conservational factors against kidney damage and metabolic disorders, are still being scrutinized, so the systematic application is not currently recommended for DKD treatment and related metabolic derangement. The dose, time length of treatment, and prolonged outcomes of the utilization of various colonies still call for further investigation; extra searches are demanded before gut microbiota therapies can be judiciously assigned for the treatment or prevention of DKD. Diet modification, lifestyle modification, and control of environmental factors are still pivotal strategies to prevent DKD progression. Our understanding of this gut-kidney crosstalk remains rudimentary, even though there is rapidly accumulating information. Additional work is needed to describe the patho-physiological elements of this interrelationship and to invent new treatments strategies to counteract a detrimental loop of DKD-gut dysbiosis which drives renal disorders to ESRD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for Cancer Research, Associate Member, No. 1075629.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qi R, China; Wen XL, China; Xiu Dong, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina (Kaunas). 2019;55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5:49-56. [PubMed] [Cited in This Article: ] |
| 3. | Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Zhang P, Colagiuri S. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 392] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 4. | Wang M, Yang Y, Liao Z. Diabetes and cancer: Epidemiological and biological links. World J Diabetes. 2020;11:227-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 66] [Cited by in F6Publishing: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Wang M, Tan Y, Shi Y, Wang X, Liao Z, Wei P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front Endocrinol (Lausanne). 2020;11:568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5345] [Cited by in F6Publishing: 4703] [Article Influence: 940.6] [Reference Citation Analysis (8)] |
| 7. | Microalbuminuria Collaborative Study Group. Microalbuminuria in type I diabetic patients. Prevalence and clinical characteristics. Diabetes Care. 1992;15:495-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, Binder C, Parving HH. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328:1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Newman DJ, Mattock MB, Dawnay AB, Kerry S, McGuire A, Yaqoob M, Hitman GA, Hawke C. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess. 2005;9:iii-ivi, xiii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 553] [Cited by in F6Publishing: 524] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 11. | Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63:225-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1163] [Cited by in F6Publishing: 1092] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 12. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1421] [Cited by in F6Publishing: 1447] [Article Influence: 160.8] [Reference Citation Analysis (43)] |
| 13. | Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am J Kidney Dis. 2016;67:483-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Nymark M, Pussinen PJ, Tuomainen AM, Forsblom C, Groop PH, Lehto M; FinnDiane Study Group. Serum lipopolysaccharide activity is associated with the progression of kidney disease in finnish patients with type 1 diabetes. Diabetes Care. 2009;32:1689-1693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 732] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 16. | Beilharz JE, Kaakoush NO, Maniam J, Morris MJ. The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain Behav Immun. 2016;57:304-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Zhang SL, Filep JG, Hohman TC, Tang SS, Ingelfinger JR, Chan JS. Molecular mechanisms of glucose action on angiotensinogen gene expression in rat proximal tubular cells. Kidney Int. 1999;55:454-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, Yukimura T, Shokoji T, Kimura S, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RG. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11:25-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Wahab NA, Yevdokimova N, Weston BS, Roberts T, Li XJ, Brinkman H, Mason RM. Role of connective tissue growth factor in the pathogenesis of diabetic nephropathy. Biochem J. 2001;359:77-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Sorokin A, Kohan DE. Physiology and pathology of endothelin-1 in renal mesangium. Am J Physiol Renal Physiol. 2003;285:F579-F589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes--part I: pathways of vascular disease in diabetes. Vascul Pharmacol. 2011;54:68-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Tilton RG, Chang K, Pugliese G, Eades DM, Province MA, Sherman WR, Kilo C, Williamson JR. Prevention of hemodynamic and vascular albumin filtration changes in diabetic rats by aldose reductase inhibitors. Diabetes. 1989;38:1258-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 110] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci U S A. 1992;89:12043-12047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 283] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest. 1997;100:115-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 361] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Davies M, Thomas GJ, Martin J, Lovett DH. The purification and characterization of a glomerular-basement-membrane-degrading neutral proteinase from rat mesangial cells. Biochem J. 1988;251:419-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Kanellis J, Fraser S, Katerelos M, Power DA. Vascular endothelial growth factor is a survival factor for renal tubular epithelial cells. Am J Physiol Renal Physiol. 2000;278:F905-F915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090-3094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Pfeilschifter J, Pignat W, Vosbeck K, Märki F. Interleukin 1 and tumor necrosis factor synergistically stimulate prostaglandin synthesis and phospholipase A2 release from rat renal mesangial cells. Biochem Biophys Res Commun. 1989;159:385-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 164] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Navarro JF, Mora C. Diabetes, inflammation, proinflammatory cytokines, and diabetic nephropathy. ScientificWorldJournal. 2006;6:908-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Rivero A, Mora C, Muros M, García J, Herrera H, Navarro-González JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond). 2009;116:479-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 749] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 33. | Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, Okamura H, Koga M, Fukuchi M, Hada T. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism. 2003;52:605-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol. 2003;284:F113-F121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224:R15-R30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 36. | Toth-Manikowski S, Atta MG. Diabetic Kidney Disease: Pathophysiology and Therapeutic Targets. J Diabetes Res. 2015;2015:697010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Fang L, Zhou Y, Cao H, Wen P, Jiang L, He W, Dai C, Yang J. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One. 2013;8:e60546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 38. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3971] [Cited by in F6Publishing: 4333] [Article Influence: 361.1] [Reference Citation Analysis (0)] |
| 39. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8203] [Cited by in F6Publishing: 7293] [Article Influence: 607.8] [Reference Citation Analysis (2)] |
| 40. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7052] [Cited by in F6Publishing: 7170] [Article Influence: 512.1] [Reference Citation Analysis (3)] |
| 41. | Tao S, Li L, Liu Y, Ren Q, Shi M, Liu J, Jiang J, Ma H, Huang Z, Xia Z, Pan J, Wei T, Wang Y, Li P, Lan T, Tang X, Zeng X, Lei S, Tang H, Ma L, Fu P. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol. 2019;56:581-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 42. | Aron-Wisnewsky J, Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12:169-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 43. | Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant. 2015;30:924-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 44. | Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant. 2016;31:737-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 45. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4095] [Cited by in F6Publishing: 4129] [Article Influence: 242.9] [Reference Citation Analysis (0)] |
| 46. | Salguero MV, Al-Obaide MAI, Singh R, Siepmann T, Vasylyeva TL. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. 2019;18:3461-3469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 47. | Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1617] [Cited by in F6Publishing: 1696] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 48. | Mudaliar H, Pollock C, Panchapakesan U. Role of Toll-like receptors in diabetic nephropathy. Clin Sci (Lond). 2014;126:685-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Mueller NT, Zhang M, Juraschek SP, Miller ER, Appel LJ. Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial. Am J Clin Nutr. 2020;111:545-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 50. | Lin MY, de Zoete MR, van Putten JP, Strijbis K. Redirection of Epithelial Immune Responses by Short-Chain Fatty Acids through Inhibition of Histone Deacetylases. Front Immunol. 2015;6:554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, Hattori M, Koga Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep. 2017;7:43522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 52. | Huang W, Guo HL, Deng X, Zhu TT, Xiong JF, Xu YH, Xu Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp Clin Endocrinol Diabetes. 2017;125:98-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 53. | Chen T, Kim CY, Kaur A, Lamothe L, Shaikh M, Keshavarzian A, Hamaker BR. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017;8:1166-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 54. | Khan S, Jena G. Sodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic rats. Food Chem Toxicol. 2014;73:127-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 55. | Lu CC, Hu ZB, Wang R, Hong ZH, Lu J, Chen PP, Zhang JX, Li XQ, Yuan BY, Huang SJ, Ruan XZ, Liu BC, Ma KL. Gut microbiota dysbiosis-induced activation of the intrarenal renin-angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacol Sin. 2020;41:1111-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Ichii O, Otsuka-Kanazawa S, Nakamura T, Ueno M, Kon Y, Chen W, Rosenberg AZ, Kopp JB. Podocyte injury caused by indoxyl sulfate, a uremic toxin and aryl-hydrocarbon receptor ligand. PLoS One. 2014;9:e108448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Addi T, Poitevin S, McKay N, El Mecherfi KE, Kheroua O, Jourde-Chiche N, de Macedo A, Gondouin B, Cerini C, Brunet P, Dignat-George F, Burtey S, Dou L. Mechanisms of tissue factor induction by the uremic toxin indole-3 acetic acid through aryl hydrocarbon receptor/nuclear factor-kappa B signaling pathway in human endothelial cells. Arch Toxicol. 2019;93:121-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Kikuchi K, Saigusa D, Kanemitsu Y, Matsumoto Y, Thanai P, Suzuki N, Mise K, Yamaguchi H, Nakamura T, Asaji K, Mukawa C, Tsukamoto H, Sato T, Oikawa Y, Iwasaki T, Oe Y, Tsukimi T, Fukuda NN, Ho HJ, Nanto-Hara F, Ogura J, Saito R, Nagao S, Ohsaki Y, Shimada S, Suzuki T, Toyohara T, Mishima E, Shima H, Akiyama Y, Ichijo M, Matsuhashi T, Matsuo A, Ogata Y, Yang CC, Suzuki C, Breeggemann MC, Heymann J, Shimizu M, Ogawa S, Takahashi N, Owada Y, Kure S, Mano N, Soga T, Wada T, Kopp JB, Fukuda S, Hozawa A, Yamamoto M, Ito S, Wada J, Tomioka Y, Abe T. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10:1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 59. | Cerdá B, Pérez M, Pérez-Santiago JD, Tornero-Aguilera JF, González-Soltero R, Larrosa M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front Physiol. 2016;7:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 60. | Ribeiro FM, Petriz B, Marques G, Kamilla LH, Franco OL. Is There an Exercise-Intensity Threshold Capable of Avoiding the Leaky Gut? Front Nutr. 2021;8:627289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 61. | Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374-2383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1237] [Cited by in F6Publishing: 1203] [Article Influence: 70.8] [Reference Citation Analysis (1)] |
| 62. | Devlin AS, Marcobal A, Dodd D, Nayfach S, Plummer N, Meyer T, Pollard KS, Sonnenburg JL, Fischbach MA. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe. 2016;20:709-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 63. | Li YJ, Chen X, Kwan TK, Loh YW, Singer J, Liu Y, Ma J, Tan J, Macia L, Mackay CR, Chadban SJ, Wu H. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J Am Soc Nephrol. 2020;31:1267-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 64. | Bohlouli J, Namjoo I, Borzoo-Isfahani M, Hojjati Kermani MA, Balouch Zehi Z, Moravejolahkami AR. Effect of probiotics on oxidative stress and inflammatory status in diabetic nephropathy: A systematic review and meta-analysis of clinical trials. Heliyon. 2021;7:e05925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 65. | Vlachou E, Ntikoudi A, Govina O, Lavdaniti M, Kotsalas N, Tsartsalis A, Dimitriadis G. Effects of Probiotics on Diabetic Nephropathy: A Systematic Review. Curr Clin Pharmacol. 2020;15:234-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Guo S, Gillingham T, Guo Y, Meng D, Zhu W, Walker WA, Ganguli K. Secretions of Bifidobacterium infantis and Lactobacillus acidophilus Protect Intestinal Epithelial Barrier Function. J Pediatr Gastroenterol Nutr. 2017;64:404-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 67. | Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut. 2003;52:988-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 409] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 68. | Bahmani F, Tajadadi-Ebrahimi M, Kolahdooz F, Mazouchi M, Hadaegh H, Jamal AS, Mazroii N, Asemi S, Asemi Z. The Consumption of Synbiotic Bread Containing Lactobacillus sporogenes and Inulin Affects Nitric Oxide and Malondialdehyde in Patients with Type 2 Diabetes Mellitus: Randomized, Double-Blind, Placebo-Controlled Trial. J Am Coll Nutr. 2016;35:506-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 69. | Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, Yoshida M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant. 2011;26:1094-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 70. | Hu ZB, Lu J, Chen PP, Lu CC, Zhang JX, Li XQ, Yuan BY, Huang SJ, Ruan XZ, Liu BC, Ma KL. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics. 2020;10:2803-2816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 50] [Article Influence: 12.5] [Reference Citation Analysis (2)] |
| 71. | Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1062] [Cited by in F6Publishing: 966] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 72. | Barba C, Soulage CO, Caggiano G, Glorieux G, Fouque D, Koppe L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 73. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1881] [Cited by in F6Publishing: 1825] [Article Influence: 152.1] [Reference Citation Analysis (0)] |
| 74. | de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, Herrema H, Ackermans M, Serlie MJM, de Brauw M, Levels JHM, Sales A, Gerdes VE, Ståhlman M, Schimmel AWM, Dallinga-Thie G, Bergman JJ, Holleman F, Hoekstra JBL, Groen A, Bäckhed F, Nieuwdorp M. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69:502-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |