Copyright
©The Author(s) 2022.
World J Diabetes. Nov 15, 2022; 13(11): 926-939
Published online Nov 15, 2022. doi: 10.4239/wjd.v13.i11.926
Published online Nov 15, 2022. doi: 10.4239/wjd.v13.i11.926
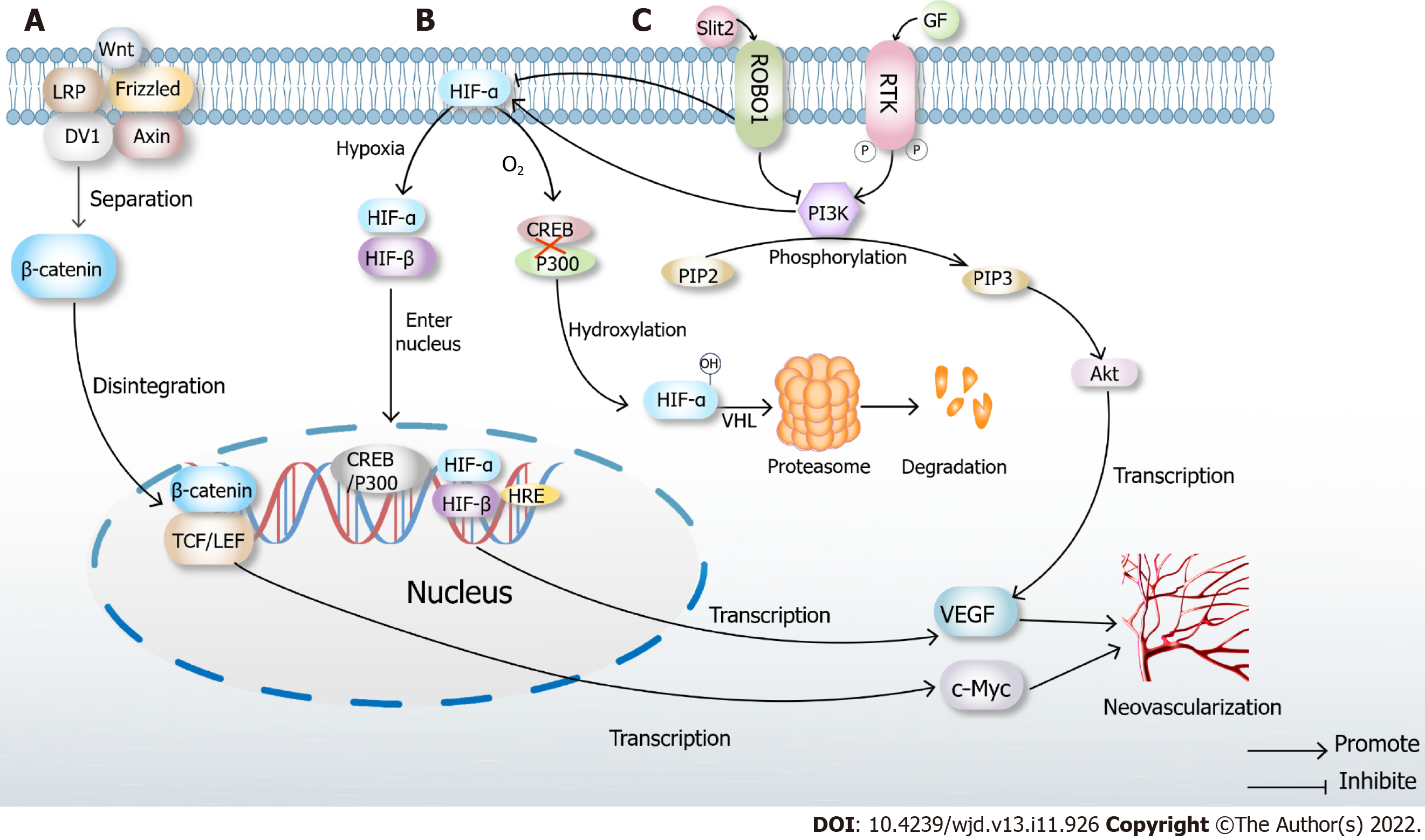
Figure 2 Classical mechanisms of neovascularization.
A: Classical Wnt pathway. When there is no Wnt signal, LRP, Frizzled, DV1 and Axin are closely combined with β-catenin. In the presence of Wnt signaling, the complex disintegrates, and β-catenin enters the nucleus, binds to TCF/LEF, transcribes multiple downstream signals such as c-Myc and ultimately promotes neovascularization; B: Under normoxic conditions, hypoxia-inducible factor (HIF)-α inhibits the binding of cAMP-response element binding protein (CREB) and P300, which causes hydroxylation of HIF, then leads to proteasome degradation and inactivation after VHL ubiquitination. When hypoxia occurs, prolyl hydroxylase domains become inactive, allowing HIF-α to migrate to the nucleus, where it dimerizes with HIF-β. The dimer binds to the hypoxia response elements of specific genes in DNA, transcribes thousands of genes such as vascular endothelial growth factor (VEGF) and promotes neovascularization; C: High glucose induces Slit2/Roundabout 1 (Robo1) binding, and Robo1 inhibits the activation of phosphatidylinositol 3kinase (PI3K) /protein kinase B (Akt) and HIF-1α/VEGF signaling pathway and inhibits angiogenesis. PI3K inhibitors also inhibit the HIF-1α/VEGF signaling pathway. After activation of the PI3K/Akt signaling pathway, a variety of cytokines including VEGF will be transcribed to promote angiogenesis.
- Citation: Cai Y, Zang GY, Huang Y, Sun Z, Zhang LL, Qian YJ, Yuan W, Wang ZQ. Advances in neovascularization after diabetic ischemia. World J Diabetes 2022; 13(11): 926-939
- URL: https://www.wjgnet.com/1948-9358/full/v13/i11/926.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i11.926