Copyright
©The Author(s) 2020.
World J Diabetes. Apr 15, 2020; 11(4): 137-149
Published online Apr 15, 2020. doi: 10.4239/wjd.v11.i4.137
Published online Apr 15, 2020. doi: 10.4239/wjd.v11.i4.137
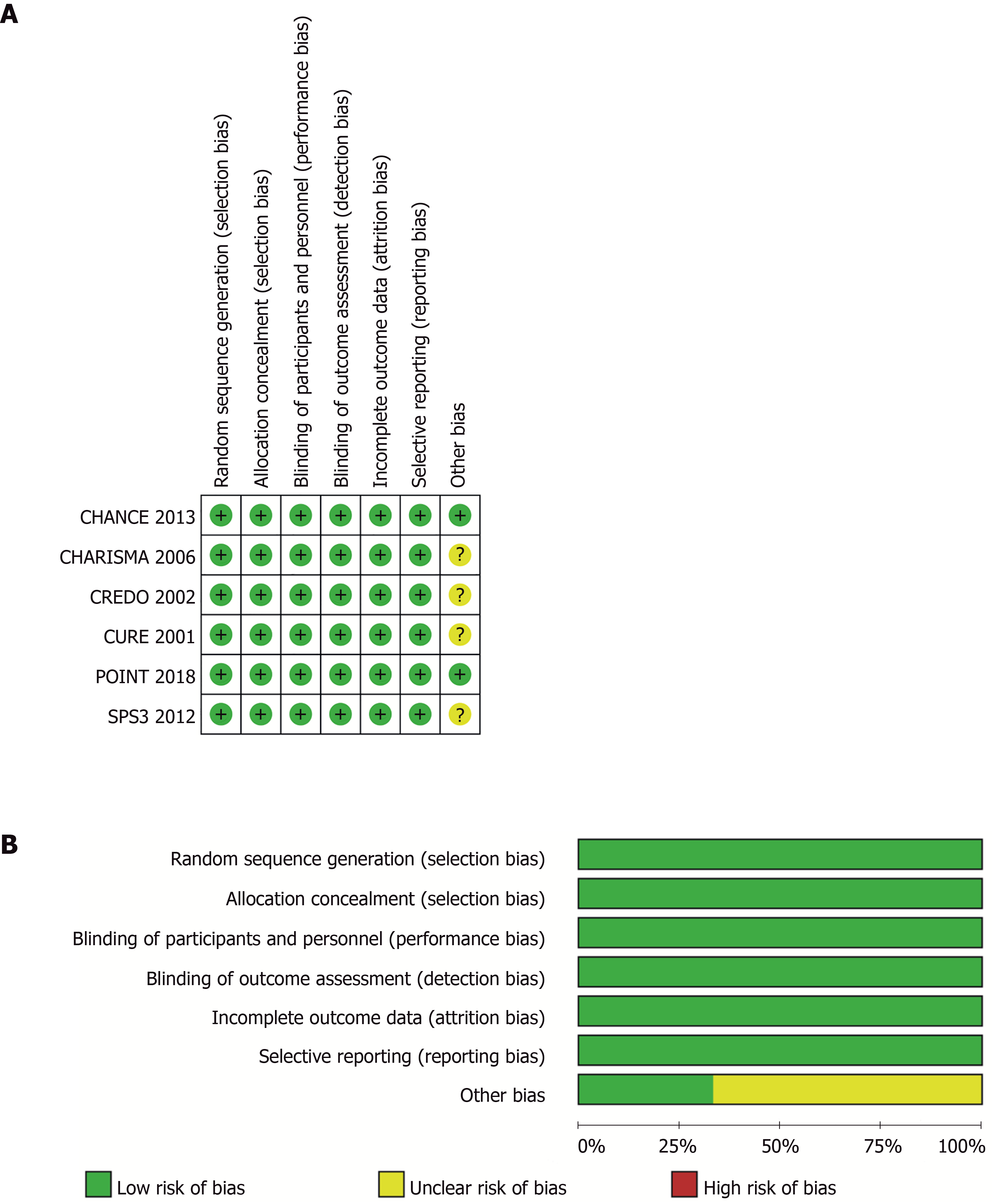
Figure 1 Risk-of-bias of included trials.
A: Judgements about each source of bias in each study; B: Review authors’ judgements regarding the risk of each source of bias. The data are presented as percentages across all the included studies and are classified as Low, Unclear, or High. We followed the recommended approach for assessing the risk of bias in studies included in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.3.0. This addresses seven specific domains: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each domain includes one or more specific entries in a “Risk of bias” table. The tool involves assigning a judgement relating to the risk of bias for that entry. This is achieved by answering a pre-specified question about the adequacy of the study in relation to the entry, such that a judgement of “Yes” indicates a low risk of bias, “No” indicates a high risk of bias, and ‘Unclear’ indicates an unclear or unknown risk of bias.
- Citation: Liang LR, Ma Q, Feng L, Qiu Q, Zheng W, Xie WX. Long-term effect of clopidogrel in patients with and without diabetes: A systematic review and meta-analysis of randomized controlled trials. World J Diabetes 2020; 11(4): 137-149
- URL: https://www.wjgnet.com/1948-9358/full/v11/i4/137.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i4.137