Published online Apr 15, 2020. doi: 10.4239/wjd.v11.i4.115
Peer-review started: December 28, 2019
First decision: January 15, 2020
Revised: March 13, 2020
Accepted: March 22, 2020
Article in press: March 22, 2020
Published online: April 15, 2020
Obesity is associated with adverse metabolic diseases including cardiovascular disease (CVD) and chronic kidney disease (CKD). These obesity-related diseases are highly associated with excess fat accumulation in adipose tissue. However, emerging evidence indicates that visceral adiposity associates more with metabolic and cardiovascular risk factors. Perirenal adipose tissue, surrounding the kidney, is originally thought to provides only mechanical support for kidney. However, more studies demonstrated perirenal adipose tissue have a closer association with renal disease than other visceral fat deposits in obesity. Additionally, perirenal adipose tissue is also an independent risk factor for CKD and even associated more with CVD. Thus, perirenal adipose tissue may be a connection of CVD with CKD. Here, we will provide an overview of the perirenal adipose tissue, a neglected visceral adipose tissue, and the roles of perirenal adipose tissue linking with CVD and CKD and highlight the perirenal adipose tissue as a potential strategy for future therapeutics against obesity-related disease.
Core tip: The presence of excess perirenal adipose tissue, a neglected visceral adipose tissue, is regarded as an independent risk factor for both chronic kidney disease and cardiovascular disease. We herein discuss the relationship of perirenal adipose tissue in chronic kidney disease and cardiovascular disease, and the potential mechanism for perirenal adipose tissue participated in obesity-related disease.
- Citation: Huang N, Mao EW, Hou NN, Liu YP, Han F, Sun XD. Novel insight into perirenal adipose tissue: A neglected adipose depot linking cardiovascular and chronic kidney disease. World J Diabetes 2020; 11(4): 115-125
- URL: https://www.wjgnet.com/1948-9358/full/v11/i4/115.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i4.115
Obesity is regarded as a risk factor for adverse metabolic diseases including hypertension, type 2 diabetes, and dyslipidemia[1-3]. These metabolic disorders cause vascular complications that primarily manifest as cardiovascular disease (CVD) and chronic kidney disease (CKD)[4,5]. Moreover, these obesity-related diseases are strongly associated with the prevalence and severity of overweight/obesity. During the development of obesity, impaired glucose homeostasis, hypertension, dyslipidemia, and changes in blood hemodynamics may contribute to the pathogenesis of obesity-related diseases[6]; these abnormal metabolic changes are mainly caused by excess fat accumulation in adipose tissue[7,8]. However, there is emerging evidence that, compared with obesity itself, body fat distribution is more closely related to the pathogeneses and development of obesity-related diseases (including CVD and obesity-related CKD)[9-11].
Adipose tissue mainly comprises a large number of adipocytes. As the main energy storage and endocrine organ, adipose tissue can maintain lipid metabolism homeostasis and energy balance by contributing to several physiological processes through secretion of various adipokines/cytokines[12,13]. Adipose tissue is traditionally classified into visceral adipose tissue (in the trunk cavity) and subcutaneous adipose tissue (under the skin), based on morphological appearance and location[14]. Compared with subcutaneous adipose tissue, visceral adipose tissue is reportedly more closely associated with metabolic and cardiovascular risk factors, such as insulin resistance, dyslipidemia, hypertension, and atherosclerosis[15,16]. Perirenal adipose tissue (PRAT) is a component of visceral adipose tissue that surrounds the kidney, which was originally presumed to only provide mechanical support for the kidney. However, PRAT has been shown to demonstrate a closer relationship to renal disease among individuals with obesity, compared with other visceral fat deposits[17]. In addition, the presence of excess PRAT has been identified as an independent risk factor for CKD and a factor associated with the development of CVD[18-22]. Thus, PRAT may serve to connect CVD with CKD among individuals with obesity. This review will provide an overview of PRAT, a neglected visceral adipose tissue, as well as the roles of PRAT in linking CVD and CKD. Moreover, it will highlight the potential for PRAT as a therapeutic target in the treatment of obesity-related diseases.
PRAT is a fat pad located in the retroperitoneal space surrounding the kidney, which fills the space between the kidney and neighboring retroperitoneal tissues, renal parenchyma, and adrenal gland; the PRAT is supported by renal fascia[23]. Fat around the renal sinus is also regarded as a component of PRAT. Notably, PRAT is the only adipose tissue that is surrounded by a multilayered fibrous membrane. Because PRAT is surrounded by fascia tissue, excess PRAT can tightly encapsulate the kidney and cause excessive renal compression. Despite its origination from preadipocytes, PRAT undergoes an unusual progressive transition from brown adipose tissue into white adipose tissue after birth[24]. Brown adipocytes form the majority of PRAT in fetuses and newborn infants (1-11 mo), while white adipocytes comprise the outermost thin layer[25]. With increasing age, only a small proportion of brown adipocyte areas remains within perirenal white adipose tissue in adults; thus, PRAT constitutes a combination of white adipose tissue and brown adipose tissue[26]. One study in Siberia showed that approximately 40% of PRAT exhibited morphology typical of brown adipocytes, while approximately 30% expressed uncoupling protein 1; these findings suggest that PRAT can be converted to brown adipose tissue in cold conditions[27].
Despite its atypical nature, PRAT has been reported to synthesize and secrete adipokines and pro-inflammatory cytokines, including adiponectin, leptin, visfatin, resistin, tumor necrosis factor-α, interleukin-6 (IL-6), and IL-1β[22]. These cytokines enter nearby kidneys and serve to regulate renal function through endocrine or paracrine pathways[28].
Anatomically, PRAT exhibits extensive vascularization, innervation, and lymph fluid drainage. The PRAT artery originates from branches of the abdominal aorta, include branches of the inferior adrenal, dorsal, and gonadal arteries[29]. Perirenal nerve fibers originate from the celiac superior mesenteric, ipsilateral inferior mesenteric, adrenal, aorticorenal, ovarian, testicular, and ipsilateral sympathetic chain ganglia[30]. Perirenal lymphatic vessels communicate with renal subcapsular lymphatic vessels and then drain into para-aortic lymph nodes[31]. These features allow interrelationships between kidney and PRAT, as well as between body function and PRAT, via secretion of adipokines and cytokines.
Because of the increasing prevalence of obesity, accurate quantification of obesity has become necessary. Traditional simple indicators (e.g., body mass index, waist circumference, and waist/hip ratio) have been widely used in clinical practice. Although these indicators are convenient and noninvasive for patients, they have obvious limitations. In particular, they are ethnicity specific and cannot accurately differentiate between visceral and subcutaneous fat, between muscle and adipose tissue; moreover, they cannot determine the regional distribution of adiposity throughout the body[8,32]. Advanced imaging techniques (e.g., computed tomography, magnetic resonance imaging, and positron emission tomography) have been utilized to evaluate body fat distribution, including PRAT thickness[33-35]. Although these techniques are more accurate, widespread clinical use of computed tomography and magnetic resonance imaging for evaluation of obesity is unsuitable because of the cost and time involved, as well as the exposure to ionizing radiation. Some noninvasive ultra-sonographic methods have been reported for assessment of fat distribution[20,36]. Armellini et al[37] first described the use of ultrasonography for direct evaluation of intra-abdominal fat deposits. Subsequently, Kawasaki et al[38]reported a more convenient method for quantification of visceral fat by measuring PRAT thickness on abdominal sonography. Measurement of PRAT can be performed as following: keep the patient in the supine position; place the ultrasound probe vertically to the abdominal lateral surface skin above the kidney; obtain the ultrasound longitudinal scan of the kidney which is almost parallel to the skin. Gender pressure of the probe should be noticed during image obtaining in order not to cause extra adipose tissue pressing. PRAT thickness was then measured from the kidney surface to inner side of abdominal musculature. Average measurement of the maximum thickness values of both sides by three times was regarded as ultrasound measure. Kawasaki et al[38] showed that PRAT thickness was positively correlated with visceral adipose tissue area and that PRAT thickness > 10 mm could be regarded as visceral fat accumulation (area > 100 cm2). In a separate analysis, our research group found that the average of PRAT thickness in healthy people was 7.95 mm and that in obese patients was 26.54 mm[17]. And PRAT was positively associated with body mass index and waist circumference; thus, sonographic evaluation of PRAT thickness could be used to assess visceral fat and predict early renal injury in patients with obesity[17]. Lamacchia et al[21] measured PRAT thickness in normal subjects (8 mm ± 2 mm for men and 5 mm ± 2 mm for women), which were validated by computed tomography measurements. With the same method, De pergola et al[39] found an average value of PRAT in obese patients with body mass index above 30 kg/m2 was 25.0 mm. Ricci et al[40] verified that PRAT was statistically different between hypertensive and nonhypertensive patients, with average value of 13.6 and 11.6 mm, respectively.
The association of obesity with CKD was first reported in 1974; obesity was linked with massive proteinuria[41]. Thereafter, many epidemiologic studies have demonstrated that kidney disease is an independent complication of obesity; these manifestations are regarded as obesity-related glomerulopathy or obesity-related kidney disease[42,43]. Klausen et al[44] screened 2696 volunteers over a period of 10 years and found that obesity was strongly associated with proteinuria. Ejerblad et al[45] observed that patients with a body mass index > 25 kg/m2 had a nearly three-fold increased risk of CKD, compared with lean patients; this risk was not affected by age. Furthermore, urinary albumin excretion was significantly greater among patients with obesity who did not have hypertension or diabetes, compared with healthy controls[17]. Compared with overall obesity, abdominal obesity was found to be more strongly associated with kidney injury[46,47]; notably, a retrospective study of 7676 patients without diabetes showed that lean patients who had abdominal obesity were at higher risk of CKD[47]. As mentioned above, PRAT has been used in measurement of visceral fat deposition. PRAT thickness was found to be markedly higher among patients with obesity who exhibited microalbuminuria, compared with healthy controls and patients with obesity who did not exhibit albuminuria[17]. The presence of excess PRAT was related to a 2.3-fold increased risk of CKD, following adjustments for body mass index and the presence of excess visceral adipose tissue[48]. Lamacchia et al[21] found that PRAT thickness could predict reduced glomerular filtration rate in patients with type 2 diabetes. Furthermore, PRAT thickness was positively associated with microalbuminuria in patients with obesity[17,49]. Based on these findings, the presence of excess PRAT is considered an independent predictor of renal injury in patients with obesity or diabetes.
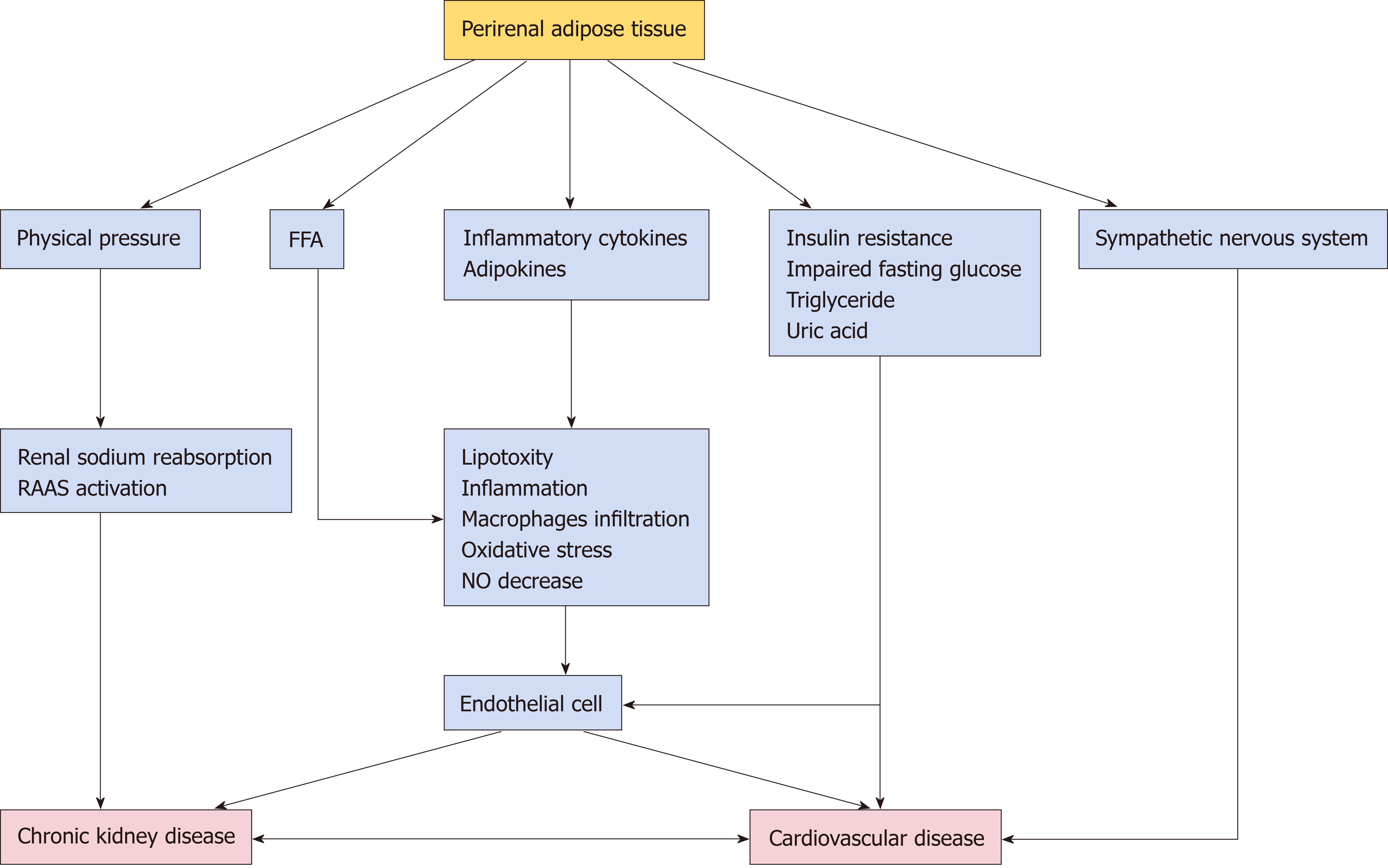
The detailed mechanisms by which PRAT initiates and exacerbates chronic renal injury remain elusive (Table 1). First, excess PRAT surrounded by renal fascia penetrates the renal sinus, obstructing renal parenchyma and vessels, promoting sodium reabsorption, and raising blood pressure. Excess PRAT encapsulates the kidney, further increasing interstitial hydrostatic pressure and reducing renal blood flow; these effects lead to hemodynamic changes, including altered renin secretion and glomerular filtration[50-53].
| Mechanistic links | Ref. | |
| Physical compression | Suppress renal parenchyma and vessel, increase renal sodium reabsorption, renin secretion and glomerular hyperfiltration | [51-54] |
| FFA | Induce renal lipotoxicity and inflammation, nitric oxide decrease, endothelial dysfunction, and renal arterial resistance | [50,55-58] |
| Cytokines | Cause endothelial dysfunction, extracellular matrix, macrophages infiltration, oxidative stress, nitric oxide decrease, renal vascular remodeling, glomerular endothelial cells proliferation and renal sympathetic nervous activity | [60-64] |
| Metabolic problems | Related with insulin resistance/hyperinsulinemia, impaired fasting glucose, triglyceride and uric acid | [20,65-67,81] |
Second, hallmarks of obesity include overproduction of free fatty acids (FFAs) and chronic inflammation. Our previous study verified that PRAT thickness is positively associated with urine albumin excretion and circulating FFAs[17]. Notably, circulating FFAs levels were found to be significantly higher in renal venous blood than in jugular venous blood, indicating that FFAs released by PRAT participate in kidney damage as direct or indirect mediators through intercellular signaling pathways[54]. Excess FFAs produced by PRAT could escape into the kidney directly or via renal vascular system based on its extensive vascularization. Adeosun et al[55] verified that FFAs from PRAT cause renal lipotoxicity through uptake of FFA metabolites, such as ceramides. Then FFAs-induced renal lipotoxicity could exacerbate chronic inflammation by increasing the metabolism of intracellular fatty acids. Furthermore, our physiology-based analysis showed that FFAs could directly impair endothelial function by enhancing oxidation of tetrahydrobiopterin; this leads to L-arginine-induced production of superoxide, rather than nitric oxide (NO), by uncoupling of endothelial NO synthase[56]. The reduction in NO level could lead to a compensatory enhancement of vascular endothelial growth factor secretion by podocytes, thereby initiating endothelial cell proliferation and permeability, which causes greater albumin leakage from glomeruli[56]. This uncoupling of glomerular vascular endothelial growth factor-NO axis could be improved by reduction of FFAs and inflammation[56,57]. Besides, FFAs-induced lipotoxicity can also increase renal arterial resistance, indicated by the high interlobar artery resistance in patients with microalbuminuria[49].
Third, excess PRAT can affect kidneys by systemic or local secretion of inflammatory cytokines[53,58]. Endothelial/vasomotor dysfunction is an early sign of vascular damage. PRAT secretes tumor necrosis factor-α, which has been shown to directly impair renal arterial endothelial dysfunction in obese swine[59]. Potential mechanism may be due to its impairment on endothelial NO balance as mentioned before. Moreover, PRAT-related inflammation and extracellular-matrix protein can be reduced by blocking plasminogen activator inhibitor-1; this mechanism was able to attenuate renal injury in obese mice[60]. Li et al[61] found that leptin secreted by excess PRAT could exacerbate renal vascular remodeling and glomerular endothelial cell proliferation by activation of the p38 MAPK pathway. These inflammatory adipocytes/cytokines could modulate cellular function via certain signaling pathways while attracting infiltrating macrophages into deposited fat, further exacerbating oxidative stress and adipocyte dysfunction[62]. Interestingly, inhibiting the levels of inflammatory cytokines including IL-6, IL-1b and tumor necrosis factor-α in PRAT through upregulation of heme oxygenase system reduced renal inflammation and ameliorated diabetic nephropathy[63]. However, precise mechanism still warrants further studies.
Finally, excess PRAT can act in a synergistic manner with metabolic risk factors to exacerbate renal damage. Patients with metabolic syndrome have greater PRAT thickness, as well as increased oxidative stress and renal microvascular proliferation[64]. Greater PRAT thickness has been associated with abnormal insulin levels, impaired fasting glucose, insulin resistance, increased triglyceride levels, and abnormal uric acid levels in patients with CKD[19,65]. One possibility is that increased FFAs and inflammatory cytokines secreted from PRAT or visceral fat impair insulin-related signaling way including PI3K/Akt inhibition and activation of protein kinase C. Insulin increases sodium retention in renal tubules, stimulates the sympathetic nervous system, and acts directly on vascular structures that contribute to kidney damage[66].
Obesity is considered a strong risk factor for CVD. Since Yudkin et al[67] first reported relationships between CKD and atherosclerosis, as well as between CKD and coronary heart disease, many epidemiological studies have confirmed a close association between CKD and CVD. The LIFE[68] and PREVEND[69] studies demonstrated that the incidence and mortality of ischemic CVD increased significantly with increasing urine albumin excretion. Furthermore, an analysis of 21050 patients in 26 countries showed that microalbuminuria was closely related to cardiovascular hazards and CVD[70]. Among individuals without diabetes and hypertension, CVD-related mortality is significantly higher in those with microalbuminuria than in those without microalbuminuria[71]. In the general population, the presence of microalbuminuria is predictive of the risks of diabetes, hypertension, and CVD[72,73]. Therefore, patients with CKD have higher risks of cardiovascular events, such as hypertension, atherosclerosis, and coronary heart disease[74]. Consistent with these observations, patients with early CKD tend to die from CVD, rather than terminal end-stage renal disease.
Visceral fat reportedly has a close association with CVD. As a component of visceral fat, the presence of excess PRAT has been recently identified as an emerging risk factor for CVD, independent of common metabolic parameters[40]. De Pergola et al[39] found that PRAT thickness was positively correlated with blood pressure in patients with overweight and obesity. Ricci et al[40] demonstrated a close association between PRAT and high blood pressure, such that PRAT can be regarded as a predictor of hypertension. Higher levels of PRAT thickness and carotid intima-media thickness were both found in HIV-infected patients with visceral adiposity, indicating that the presence of excess PRAT is associated with atherosclerosis[75]. Notably, the associations of PRAT with carotid intima-media thickness and diverse metabolic risk factors were present even in children[76].
As a metabolic risk factor related to CVD, PRAT associated with diabetes or dyslipidemia may also indirectly affect CVD. A cross-sectional study showed that the presence of excess PRAT was independently associated with hyperinsulinemia and insulin resistance in patients with obesity, independent of other anthropometric and metabolic parameters; this finding indicated that the presence of excess PRAT is a strong marker of insulin resistance[65,77]. PRAT development and remodeling have also been associated with metabolic syndrome[21,78]. In patients with CKD, the PRAT thickness was significantly correlated with metabolic risks, such as abnormal triglycerides and uric acid levels; patients with stages 4 and 5 CKD had the greatest PRAT thickness[19]. Excess PRAT is related to reductions in glomerular filtration rate, regardless of other indices of adiposity, in patients with hypertension[20]. Considering the above pathological processes, the presence of excess PRAT is believed to contribute to dysmetabolism-associated CVD. PRAT is potentially related to epicardial fat because both exhibit mesothelial layers similar to those of visceral organs, which are enriched in white adipose tissue progenitors that produce adipocytes[65]. This finding strengthens the hypothesis that excess PRAT is a predictor of the risk of CVD, because epicardial fat has been regarded as a risk factor for CVD that predicts the tendency of cardiac dysfunction.
Although there is a potential link between CKD and CVD, the mechanisms remain unclear (Figure 1). Potential mechanisms for perirenal fat-mediated CVD regulation are closely associated with anatomical, physiological, and localization features.
Importantly, efferent nerves have been found to innervate PRAT, while afferent nerves have been found in adipose tissue; the physiological functions of PRAT neural activity are presumably related to these anatomical characteristics. Afferent nerves of adipose tissue might control the sympathetic nervous system by forming a negative feedback loop (i.e., a reflex)[24]. Renal sympathetic outflow is increased by enhancement of afferent signals from fat deposits, followed by the elevation of arterial blood pressure; this reflex phenomenon is known as the “adipose afferent reflex”[24]. Tanida et al[79] injected leptin into PRAT; this resulted in activation of adipose afferent reflex without affecting the serum levels of sympathetic-activating substances, suggesting that PRAT may directly regulate the cardiovascular system. Excess PRAT is presumed to contribute to increased hydrostatic pressure and activation of renin-angiotensin-aldosterone system through the compression of blood vessels, lymphatic vessels, and ureters; this may lead to the development of hypertension and atherosclerosis[80]. Additionally, adipokines/cytokines synthesized by excess PRAT could regulate functions of the cardiovascular system via autocrine, paracrine, and endocrine pathways using similar mechanisms to those described for CKD.
Moreover, endothelial injury is typically regarded as the main initiating event for atherosclerosis and CVD. The Danish Diabetes Center proposed the Steno hypothesis, in which the emergence of microalbuminuria indicates the existence of extensive systemic endothelial dysfunction and increased systemic vascular permeability[81]. This may explain why CKD is an indicator of increased risk of CVD and can strongly predict the occurrence of CVD. Importantly, microalbuminuria reflects the presence of microvascular disease and early damage to renal function; it is also an independent risk factor for macrovascular disease and CVD, including the occurrence and development of cardiovascular events.
According to current literature, lifestyle intervention including diet control and suitable exercise should be the first line of treatment of obesity. Energy‐restricted diets effectively reduce fat mass and impact cardiometabolic profiles[82]. Parrish et al[83] reported that a fish oil diet reduced PRAT and limited fat hypertrophy. Interestingly, altering meal frequency to twice a day has been demonstrated to combat obesity by reducing PRAT[84]. Additionally, intensity interval exercise (especially short time-high frequency) is proved to have beneficial effects on the body composition including reducing PRAT[85]. Bariatric surgery is another effective intervention for morbidly obese patients. Ricci et al[40] reported that PRAT in morbidly obese patients (body mass index > 35 kg/m2) significantly was reduced accompanied by a significant reduction in blood pressure after sleeve‐gastrectomy.
As mentions above, PRAT is different from traditional visceral fat because it has characteristic of both brown- and white-fat. Jespersen et al[86] characterized the perirenal region of humans and identified the presence of dormant brown adipose tissue in the PRAT. This property suggests that reactivating dormant BAT into active BAT by cold exposure or β3-adrenoceptors stimulation may be an promising strategy for combatting obesity-related metabolic disease[27,87]. However, identifying specific drugs to induce white-to-brown adipocyte and delivering them locally or selectively to PRAT require technological advances and further research.
PRAT is a component of visceral fat that is strongly associated with adverse cardiometabolic risk factors for both CKD and CVD. Potential related mechanisms have been summarized in this review, with the aim of providing new insights and potential therapeutic targets for anti-obesity-related cardiovascular and kidney disease therapies. Further studies are needed to characterize the important roles of PRAT in the interactions between CKD and CVD. Drugs used to target CVD risk factors may influence PRAT in a manner that aids in disease prevention. The clinical implications of reducing PRAT accumulation by balanced diet, intermittent exercise, or other therapeutic interventions (e.g., burning excess energy through conversion of white adipose tissue to brown adipose tissue) with regard to CVD prevention remain unclear and should be established in future studies.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Association for the Study of Diabetes (310808).
Specialty type: Endocrinology and metabolism
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reggiani GM, Liu HK S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Landrier JF, Derghal A, Mounien L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 2. | Ghasemi A, Hashemy SI, Azimi-Nezhad M, Dehghani A, Saeidi J, Mohtashami M. The cross-talk between adipokines and miRNAs in health and obesity-mediated diseases. Clin Chim Acta. 2019;499:41-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Heinonen S, Jokinen R, Rissanen A, Pietiläinen KH. White adipose tissue mitochondrial metabolism in health and in obesity. Obes Rev. 2020;21:e12958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 4. | Echouffo-Tcheugui JB, Short MI, Xanthakis V, Field P, Sponholtz TR, Larson MG, Vasan RS. Natural History of Obesity Subphenotypes: Dynamic Changes Over Two Decades and Prognosis in the Framingham Heart Study. J Clin Endocrinol Metab. 2019;104:738-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Katsiki N, Anagnostis P, Kotsa K, Goulis DG, Mikhailidis DP. Obesity, Metabolic Syndrome and the Risk of Microvascular Complications in Patients with Diabetes mellitus. Curr Pharm Des. 2019;25:2051-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Lopes LL, Bressan J, Peluzio MDCG, Hermsdorff HHM. LINE-1 in Obesity and Cardiometabolic Diseases: A Systematic Review. J Am Coll Nutr. 2019;38:478-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Martin-Calvo N, Moreno-Galarraga L, Martinez-Gonzalez MA. Association between Body Mass Index, Waist-to-Height Ratio and Adiposity in Children: A Systematic Review and Meta-Analysis. Nutrients. 2016;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Deurenberg P, Yap M. The assessment of obesity: methods for measuring body fat and global prevalence of obesity. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Ferreira TDS, Barreto Silva MI, da Costa MS, Pontes KSDS, Castro FG, Antunes VP, Rosina KTC, Menna Barreto APM, Souza E, Klein MRST. High abdominal adiposity and low phase angle in overweight renal transplant recipients. Clin Transplant. 2019;33:e13654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Mulligan AA, Lentjes MAH, Luben RN, Wareham NJ, Khaw KT. Changes in waist circumference and risk of all-cause and CVD mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovasc Disord. 2019;19:238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Bi X, Tey SL, Leong C, Quek R, Loo YT, Henry CJ. Correlation of adiposity indices with cardiovascular disease risk factors in healthy adults of Singapore: a cross-sectional study. BMC Obes. 2016;3:33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15:507-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 13. | Zhu Q, Scherer PE. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat Rev Nephrol. 2018;14:105-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 518] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 15. | Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21:345-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O'Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 471] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 17. | Sun X, Han F, Miao W, Hou N, Cao Z, Zhang G. Sonographic evaluation of para- and perirenal fat thickness is an independent predictor of early kidney damage in obese patients. Int Urol Nephrol. 2013;45:1589-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Karastergiou K, Fried SK. Multiple adipose depots increase cardiovascular risk via local and systemic effects. Curr Atheroscler Rep. 2013;15:361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | D'Marco L, Salazar J, Cortez M, Salazar M, Wettel M, Lima-Martínez M, Rojas E, Roque W, Bermúdez V. Perirenal fat thickness is associated with metabolic risk factors in patients with chronic kidney disease. Kidney Res Clin Pract. 2019;38:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Geraci G, Zammuto MM, Mattina A, Zanoli L, Geraci C, Granata A, Nardi E, Fatuzzo PM, Cottone S, Mulè G. Para-perirenal distribution of body fat is associated with reduced glomerular filtration rate regardless of other indices of adiposity in hypertensive patients. J Clin Hypertens (Greenwich). 2018;20:1438-1446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Lamacchia O, Nicastro V, Camarchio D, Valente U, Grisorio R, Gesualdo L, Cignarelli M. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant. 2011;26:892-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Liu BX, Sun W, Kong XQ. Perirenal Fat: A Unique Fat Pad and Potential Target for Cardiovascular Disease. Angiology. 2019;70:584-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Marx WJ, Patel SK. Renal fascia: its radiographic importance. Urology. 1979;13:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol (1985). 2012;112:1008-1014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Tanuma Y, Ohata M, Ito T, Yokochi C. Possible function of human brown adipose tissue as suggested by observation on perirenal brown fats from necropsy cases of variable age groups. Arch Histol Jpn. 1976;39:117-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Tanuma Y, Tamamoto M, Ito T, Yokochi C. The occurrence of brown adipose tissue in perirenal fat in Japanese. Arch Histol Jpn. 1975;38:43-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Efremova A, Senzacqua M, Venema W, Isakov E, Di Vincenzo A, Zingaretti MC, Protasoni M, Thomski M, Giordano A, Cinti S. A large proportion of mediastinal and perirenal visceral fat of Siberian adult people is formed by UCP1 immunoreactive multilocular and paucilocular adipocytes. J Physiol Biochem. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Lau WB, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma XL, Ouchi N. Role of Adipokines in Cardiovascular Disease. Circ J. 2017;81:920-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 29. | Meyers MA, Friedenberg RM, King MC, Meng CH. The significance of the renal capsular arteries. Br J Radiol. 1967;40:949-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Czaja K, Lakomy M, Kaleczyc J, Barb CR, Rampacek GB, Kraeling RR. Leptin receptors, NPY, and tyrosine hydroxylase in autonomic neurons supplying fat depots in a pig. Biochem Biophys Res Commun. 2002;293:1138-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Kim JH, Han EH, Jin ZW, Lee HK, Fujimiya M, Murakami G, Cho BH. Fetal topographical anatomy of the upper abdominal lymphatics: its specific features in comparison with other abdominopelvic regions. Anat Rec (Hoboken). 2012;295:91-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Jones DW, Kim JS, Andrew ME, Kim SJ, Hong YP. Body mass index and blood pressure in Korean men and women: the Korean National Blood Pressure Survey. J Hypertens. 1994;12:1433-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 130] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Graffy PM, Pickhardt PJ. Quantification of hepatic and visceral fat by CT and MR imaging: relevance to the obesity epidemic, metabolic syndrome and NAFLD. Br J Radiol. 2016;89:20151024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Seabolt LA, Welch EB, Silver HJ. Imaging methods for analyzing body composition in human obesity and cardiometabolic disease. Ann N Y Acad Sci. 2015;1353:41-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Favre G, Grangeon-Chapon C, Raffaelli C, François-Chalmin F, Iannelli A, Esnault V. Perirenal fat thickness measured with computed tomography is a reliable estimate of perirenal fat mass. PLoS One. 2017;12:e0175561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Tripathy P, Sahu A, Sahu M, Nagy A. Ultrasonographic evaluation of intra-abdominal fat distribution and study of its influence on subclinical atherosclerosis in women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2017;217:18-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Armellini F, Zamboni M, Rigo L, Todesco T, Bergamo-Andreis IA, Procacci C, Bosello O. The contribution of sonography to the measurement of intra-abdominal fat. J Clin Ultrasound. 1990;18:563-567. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Kawasaki S, Aoki K, Hasegawa O, Numata K, Tanaka K, Shibata N, Shimada S, Okamura A, Terauchi Y. Sonographic evaluation of visceral fat by measuring para- and perirenal fat. J Clin Ultrasound. 2008;36:129-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | De Pergola G, Campobasso N, Nardecchia A, Triggiani V, Caccavo D, Gesualdo L, Silvestris F, Manno C. Para- and perirenal ultrasonographic fat thickness is associated with 24-hours mean diastolic blood pressure levels in overweight and obese subjects. BMC Cardiovasc Disord. 2015;15:108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Ricci MA, Scavizzi M, Ministrini S, De Vuono S, Pucci G, Lupattelli G. Morbid obesity and hypertension: The role of perirenal fat. J Clin Hypertens (Greenwich). 2018;20:1430-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Weisinger JR, Kempson RL, Eldridge FL, Swenson RS. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med. 1974;81:440-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 216] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 934] [Cited by in F6Publishing: 925] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 43. | Othman M, Kawar B, El Nahas AM. Influence of obesity on progression of non-diabetic chronic kidney disease: a retrospective cohort study. Nephron Clin Pract. 2009;113:c16-c23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Klausen KP, Parving HH, Scharling H, Jensen JS. Microalbuminuria and obesity: impact on cardiovascular disease and mortality. Clin Endocrinol (Oxf). 2009;71:40-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 426] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 46. | Bonnet F, Marre M, Halimi JM, Stengel B, Lange C, Laville M, Tichet J, Balkau B. Larger waist circumference is a predictive factor for the occurrence of microalbuminuria in a non-diabetic population. Arch Mal Coeur Vaiss. 2006;99:660-662. [PubMed] [Cited in This Article: ] |
| 47. | Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE; PREVEND Study Group. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis. 2003;41:733-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 245] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 48. | Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 49. | Han F, Hou N, Miao W, Sun X. Correlation of ultrasonographic measurement of intrarenal arterial resistance index with microalbuminuria in nonhypertensive, nondiabetic obese patients. Int Urol Nephrol. 2013;45:1039-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Rea DJ, Heimbach JK, Grande JP, Textor SC, Taler SJ, Prieto M, Larson TS, Cosio FG, Stegall MD. Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int. 2006;70:1636-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 52. | Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 53. | Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28 Suppl 4:S58-S65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 54. | Hou N, Han F, Wang M, Huang N, Zhao J, Liu X, Sun X. Perirenal fat associated with microalbuminuria in obese rats. Int Urol Nephrol. 2014;46:839-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Adeosun SO, Gordon DM, Weeks MF, Moore KH, Hall JE, Hinds TD, Stec DE. Loss of biliverdin reductase-A promotes lipid accumulation and lipotoxicity in mouse proximal tubule cells. Am J Physiol Renal Physiol. 2018;315:F323-F331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Sun X, Yu Y, Han L. High FFA levels related to microalbuminuria and uncoupling of VEGF-NO axis in obese rats. Int Urol Nephrol. 2013;45:1197-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Hou N, Huang N, Han F, Zhao J, Liu X, Sun X. Protective effects of adiponectin on uncoupling of glomerular VEGF-NO axis in early streptozotocin-induced type 2 diabetic rats. Int Urol Nephrol. 2014;46:2045-2051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Katsiki N, Athyros VG, Mikhailidis DP. Abnormal Peri-Organ or Intra-organ Fat (APIFat) Deposition: An Underestimated Predictor of Vascular Risk? Curr Vasc Pharmacol. 2016;14:432-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Ma S, Zhu XY, Eirin A, Woollard JR, Jordan KL, Tang H, Lerman A, Lerman LO. Perirenal Fat Promotes Renal Arterial Endothelial Dysfunction in Obese Swine through Tumor Necrosis Factor-α. J Urol. 2016;195:1152-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Liu Y, Wang L, Luo M, Chen N, Deng X, He J, Zhang L, Luo P, Wu J. Inhibition of PAI-1 attenuates perirenal fat inflammation and the associated nephropathy in high-fat diet-induced obese mice. Am J Physiol Endocrinol Metab. 2019;316:E260-E267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Li H, Li M, Liu P, Wang Y, Zhang H, Li H, Yang S, Song Y, Yin Y, Gao L, Cheng S, Cai J, Tian G. Telmisartan Ameliorates Nephropathy in Metabolic Syndrome by Reducing Leptin Release From Perirenal Adipose Tissue. Hypertension. 2016;68:478-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Michaud A, Drolet R, Noël S, Paris G, Tchernof A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism. 2012;61:689-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Ndisang JF, Jadhav A, Mishra M. The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in Zucker diabetic fatty rats. PLoS One. 2014;9:e87936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Li Z, Woollard JR, Wang S, Korsmo MJ, Ebrahimi B, Grande JP, Textor SC, Lerman A, Lerman LO. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am J Physiol Renal Physiol. 2011;301:F1078-F1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Manno C, Campobasso N, Nardecchia A, Triggiani V, Zupo R, Gesualdo L, Silvestris F, De Pergola G. Relationship of para- and perirenal fat and epicardial fat with metabolic parameters in overweight and obese subjects. Eat Weight Disord. 2019;24:67-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Rao A, Pandya V, Whaley-Connell A. Obesity and insulin resistance in resistant hypertension: implications for the kidney. Adv Chronic Kidney Dis. 2015;22:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Yudkin JS, Forrest RD, Jackson CA. Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Islington Diabetes Survey. Lancet. 1988;2:530-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 566] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 68. | Wachtell K, Olsen MH, Dahlöf B, Devereux RB, Kjeldsen SE, Nieminen MS, Okin PM, Papademetriou V, Mogensen CE, Borch-Johnsen K, Ibsen H. Microalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. J Hypertens. 2002;20:405-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 69. | Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777-1782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1126] [Cited by in F6Publishing: 1063] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 70. | Tebbe U, Bramlage P, Thoenes M, Paar WD, Danchin N, Volpe M, Schrader J, Noll G, Burnier M, Böhm M. Prevalence of microalbuminuria and its associated cardiovascular risk: German and Swiss results of the recent global i-SEARCH survey. Swiss Med Wkly. 2009;139:473-480. [PubMed] [Cited in This Article: ] |
| 71. | Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1708] [Cited by in F6Publishing: 1689] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 72. | Kumar Jha P, Ete T, Malviya A, Kumar Das C, Saha SK, Nath D, Kapoor M, Mishra A. Microalbuminuria: Correlation With Prevalence and Severity of Coronary Artery Disease in Non-Diabetics. J Clin Med Res. 2017;9:838-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Yuyun MF, Khaw KT, Luben R, Welch A, Bingham S, Day NE, Wareham NJ; European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Microalbuminuria independently predicts all-cause and cardiovascular mortality in a British population: The European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Int J Epidemiol. 2004;33:189-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 74. | Bajaj NS, Singh A, Zhou W, Gupta A, Fujikura K, Byrne C, Harms HJ, Osborne MT, Bravo P, Andrikopolou E, Divakaran S, Bibbo CF, Hainer J, Skali H, Taqueti V, Steigner M, Dorbala S, Charytan DM, Prabhu SD, Blankstein R, Deo RC, Solomon SD, Di Carli MF. Coronary Microvascular Dysfunction, Left Ventricular Remodeling, and Clinical Outcomes in Patients With Chronic Kidney Impairment. Circulation. 2020;141:21-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 75. | Grima P, Guido M, Zizza A, Chiavaroli R. Sonographically measured perirenal fat thickness: an early predictor of atherosclerosis in HIV-1-infected patients receiving highly active antiretroviral therapy? J Clin Ultrasound. 2010;38:190-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Bassols J, Martínez-Calcerrada JM, Prats-Puig A, Carreras-Badosa G, Xargay-Torrent S, Lizarraga-Mollinedo E, Feliu-Alsina M, Riera-Pérez E, Osiniri I, de Zegher F, Ibáñez L, López-Bermejo A. Perirenal fat is related to carotid intima-media thickness in children. Int J Obes (Lond). 2018;42:641-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 78. | Roever L, Resende ES, Veloso FC, Diniz AL, Penha-Silva N, Casella-Filho A, Dourado PM, Chagas AC. Perirenal Fat and Association With Metabolic Risk Factors: The Uberlândia Heart Study. Medicine (Baltimore). 2015;94:e1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Tanida M, Iwashita S, Ootsuka Y, Terui N, Suzuki M. Leptin injection into white adipose tissue elevates renal sympathetic nerve activity dose-dependently through the afferent nerves pathway in rats. Neurosci Lett. 2000;293:107-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Grima P, Guido M, Chiavaroli R, Zizza A. Ultrasound-assessed perirenal fat is related to increased ophthalmic artery resistance index in HIV-1 patients. Cardiovasc Ultrasound. 2010;8:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 986] [Cited by in F6Publishing: 941] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 82. | Mateo-Gallego R, Marco-Benedí V, Perez-Calahorra S, Bea AM, Baila-Rueda L, Lamiquiz-Moneo I, de Castro-Orós I, Cenarro A, Civeira F. Energy-restricted, high-protein diets more effectively impact cardiometabolic profile in overweight and obese women than lower-protein diets. Clin Nutr. 2017;36:371-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Parrish CC, Pathy DA, Angel A. Dietary fish oils limit adipose tissue hypertrophy in rats. Metabolism. 1990;39:217-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Yan H, Cao S, Li Y, Zhang H, Liu J. Reduced meal frequency alleviates high-fat diet-induced lipid accumulation and inflammation in adipose tissue of pigs under the circumstance of fixed feed allowance. Eur J Nutr. 2020;59:595-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Zhao SQ, Shi HJ, Zheng NN. [Effects of different intensity interval exercise of 6 weeks on body composition of obese rats]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019;35:326-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 86. | Jespersen NZ, Feizi A, Andersen ES, Heywood S, Hattel HB, Daugaard S, Peijs L, Bagi P, Feldt-Rasmussen B, Schultz HS, Hansen NS, Krogh-Madsen R, Pedersen BK, Petrovic N, Nielsen S, Scheele C. Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Mol Metab. 2019;24:30-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 87. | Warner A, Kjellstedt A, Carreras A, Böttcher G, Peng XR, Seale P, Oakes N, Lindén D. Activation of β3-adrenoceptors increases in vivo free fatty acid uptake and utilization in brown but not white fat depots in high-fat-fed rats. Am J Physiol Endocrinol Metab. 2016;311:E901-E910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |