Published online Jul 15, 2019. doi: 10.4239/wjd.v10.i7.414
Peer-review started: February 20, 2019
First decision: May 8, 2019
Revised: May 24, 2019
Accepted: June 11, 2019
Article in press: June 11, 2019
Published online: July 15, 2019
Processing time: 147 Days and 3.8 Hours
Maturity-onset diabetes of the young (MODY) is the most common form of monogenic diabetes. The disease is transmitted in autosomal dominant mode and diabetes is usually diagnosed before age 25 year. MODY 3 is caused by mutation of hepatocyte nuclear factor (HNF) 1A genes and is the most common MODY subtype. Diagnosis of MODY 3 is crucial since glycemic control can be accomplished by very low dose of sulfonylurea. In this report we described a Thai MODY 3 patient who had excellence plasma glucose control by treating with glicazide 20 mg per day and insulin therapy can be discontinued.
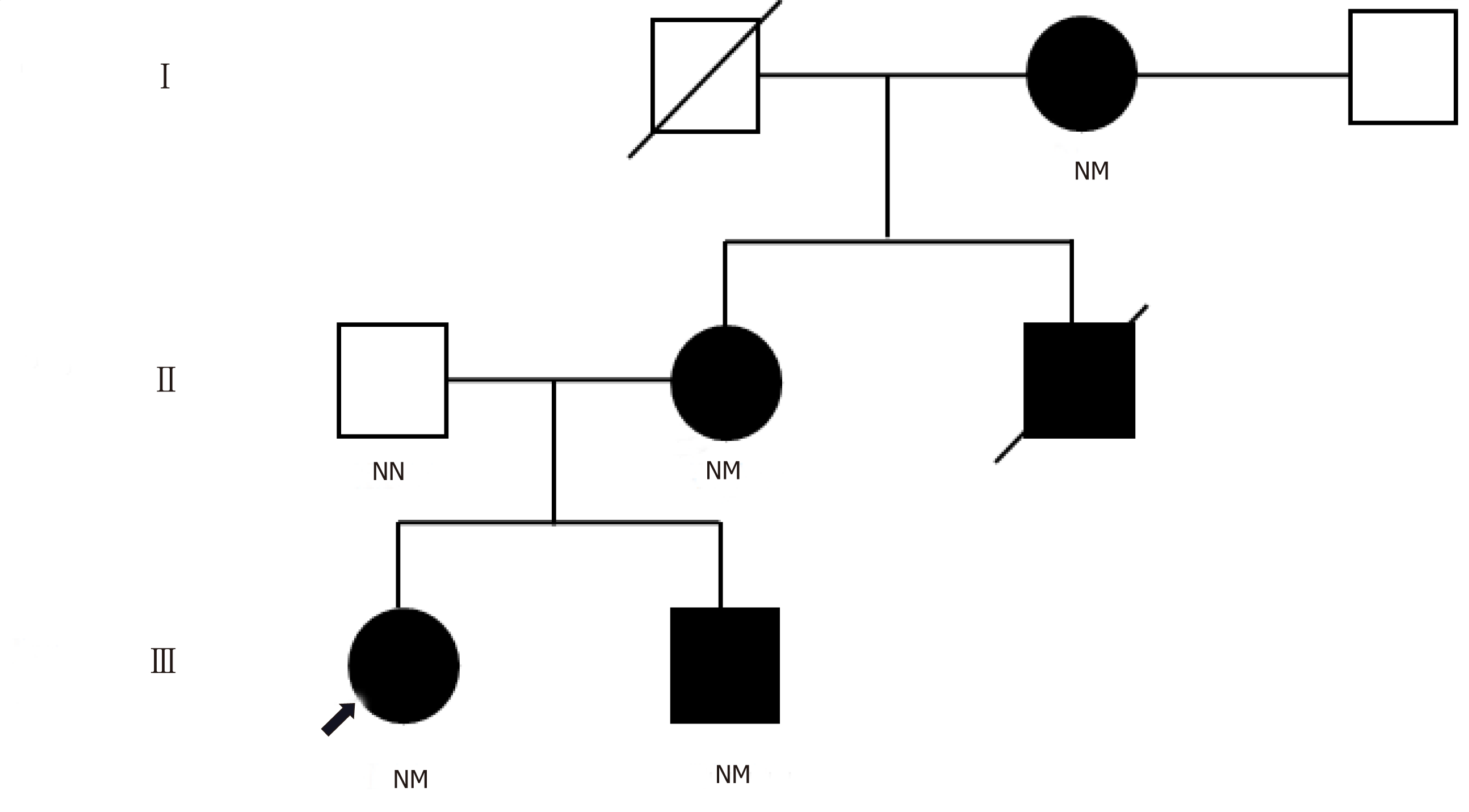
A 31-year-old woman was diagnosed diabetes mellitus at 14 years old. The disease was transmitted from her grandmother and mother compatible with autosomal dominant inheritance. Sanger sequencing of proband’s DNA identified mutation of HNF1A at codon 203 which changed amino acid from arginine to cysteine (R203C). This mutation was carried only by family members who have diabetes. The patient has been treated effectively with a combination of oral hypoglycemic agents and must include a very low dose of glicazide (20 mg/d). Insulin therapy was successfully discontinued.
We demonstrated a first case of pharmacogenetics in Thai MODY 3 patient. Our findings underscore the essential role of molecular genetics in diagnosis and guidance of appropriate treatment of diabetes mellitus in particular patient.
Core tip: Maturity-onset diabetes of the young (MODY) is the most common form of diabetes in patients diagnosed under the age of 25. In addition, MODY is characterized by autosomal dominant inheritance. We report a R203C mutation in the HNF1A causing MODY type 3. The genetic diagnosis is implicated to alter SU treatment. This study revealed that excellent glycemic control in this patient could be achieved by very low dose SU. Furthermore, this is the first report of exceptional response to treatment with SU in Thai MODY3.
- Citation: Plengvidhya N, Tangjittipokin W, Teerawattanapong N, Narkdontri T, Yenchitsomanus PT. HNF1A mutation in a Thai patient with maturity-onset diabetes of the young: A case report. World J Diabetes 2019; 10(7): 414-420
- URL: https://www.wjgnet.com/1948-9358/full/v10/i7/414.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i7.414
Maturity-onset diabetes of the young (MODY) is the most common type of monogenic diabetes, it is inherited in an autosomal dominant manner, and it is normally diagnosed before 25 years of age. To date, at least 15 subtypes of MODY caused by mutations of 15 different genes have been identified[1,2]. Thus, the clinical hete-rogeneity of MODY is explained by its genetic heterogeneity[3]. MODY3 is caused by mutation of hepatocyte nuclear factor 1A (HNF1A), which encodes a transcription factor that regulates functions of several proteins, including amylin, insulin, GLUT2, and L-pyruvate kinase, that are important for glucose metabolism and insulin secretion. HNF1A dysfunction are leading to Diabetes development and imbalance of insulin in patients. More than 350 mutations of HNF1A have been identified, and MODY3 is the most common MODY subtype among Caucasians[4]. In contrast, Asians most commonly have MODY-X or MODY subtype without identified genetic cause[5-10]. Identification of MODY3 is very important, because pancreatic β-cells exhibited hyperexcitability in this subtype in response to treatment with sulfonylurea (SU)[11]. The Siriraj Center of Research Excellence for Diabetes and Obesity (SiCORE-DO) discovered 3 different HNF1A mutations, including R203C, G554fsX556, and P475L, in 3 unrelated MODY pedigree[12-14]. Here, we report a Thai MODY3 patient carrying HNF1A R203C mutation that exhibited outstanding diabetes control with low-dose glicazide, which is a short-acting second-generation SU. Rapid deterioration of her glycemic control was observed after withdrawal of SU. The purpose of this report is to present alteration of drug treatment in patient by genetic diagnosis.
A 31-year-old Thai woman came to Siriraj Diabetes Center, Siriraj Hospital, Bangkok, Thailand for her diabetes management.
The patient has been following up every 3 months at the Siriraj Diabetes Center, Siriraj Hospital, Bangkok, Thailand. Currently, she was 44 years old and treated with glicazide 20 mg/d. She has excellence glycemic control without diabetic compli-cations. Laboratory assessment included fasting plasma glucose (FPG) 78 mg/dL, hemoglobin A1c (HbA1c) 6.7%, serum creatinine (0.56 mg/dL), total cholesterol (TC) 173 mg/dL, high-density lipoprotein (HDL) 99 mg/dL, low-density lipoprotein (LDL) 62.6 mg/dL, and triglycerides (TG) 57 mg/dL.
The patient was first seen at Siriraj Diabetes Center when she was 31 years old and diabetes was diagnosed at age 14.
Her mother and brother were diagnosed with diabetes at age 17 and 13, respectively. There was no history of diabetic ketoacidosis, and glycemic control could be achieved without insulin treatment for more than 5 years after diabetes diagnosis in all 3 patients.
The patient’s body mass index (BMI), waist-to-hip ratio, and blood pressure was 19.43 kg/m2, 0.83, and 120/70 mmHg, respectively (Table 1).
| Characteristics | Values |
| Age (yr) | 31 |
| Age at onset (yr) | 14 |
| Duration (yr) | 17 |
| BMI (kg/m2) | 19.43 |
| Waist circumference (cm) | 77 |
| Waist-to-hip ratio | 0.83 |
| Systolic BP (mmHg) | 120 |
| FPG (mg/dL) | 126 |
| HbA1c (%) | 9.5 |
| Serum creatinine (mg/dL) | 0.6 |
| Total cholesterol (mg/dL) | 156 |
| Total triglycerides (mg/dL) | 55 |
| LDL (mg/dL) | 90 |
| HDL (mg/dL) | 71.0 |
Laboratory assessments at her first visit to Siriraj Diabetes Center included FPG 126 mg/dL, HbA1c 9.5%, serum creatinine (0.6 mg/dL), TC 156 mg/dL, HDL 71 mg/dL, LDL 90 mg/dL, and total TG 55 mg/dL.
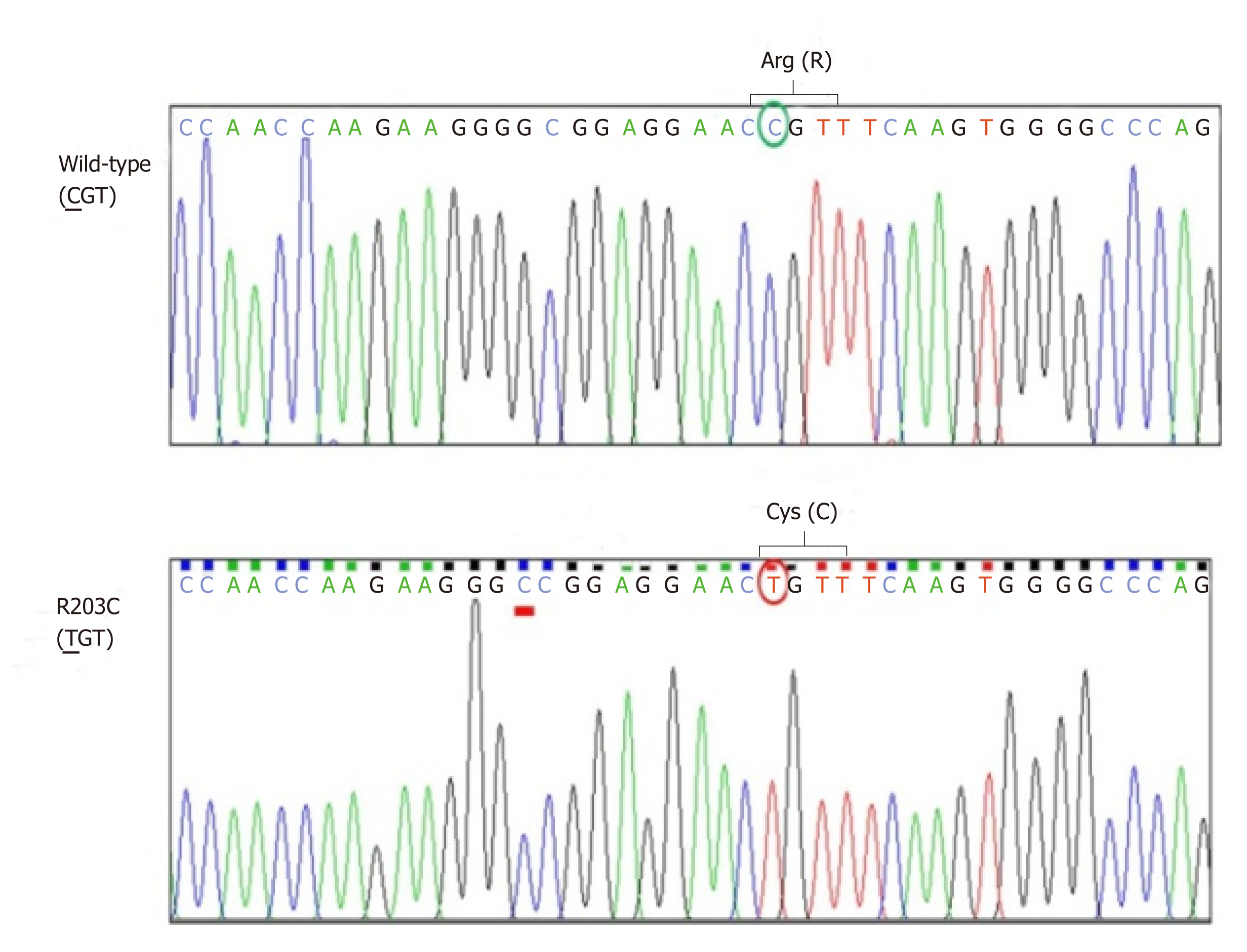
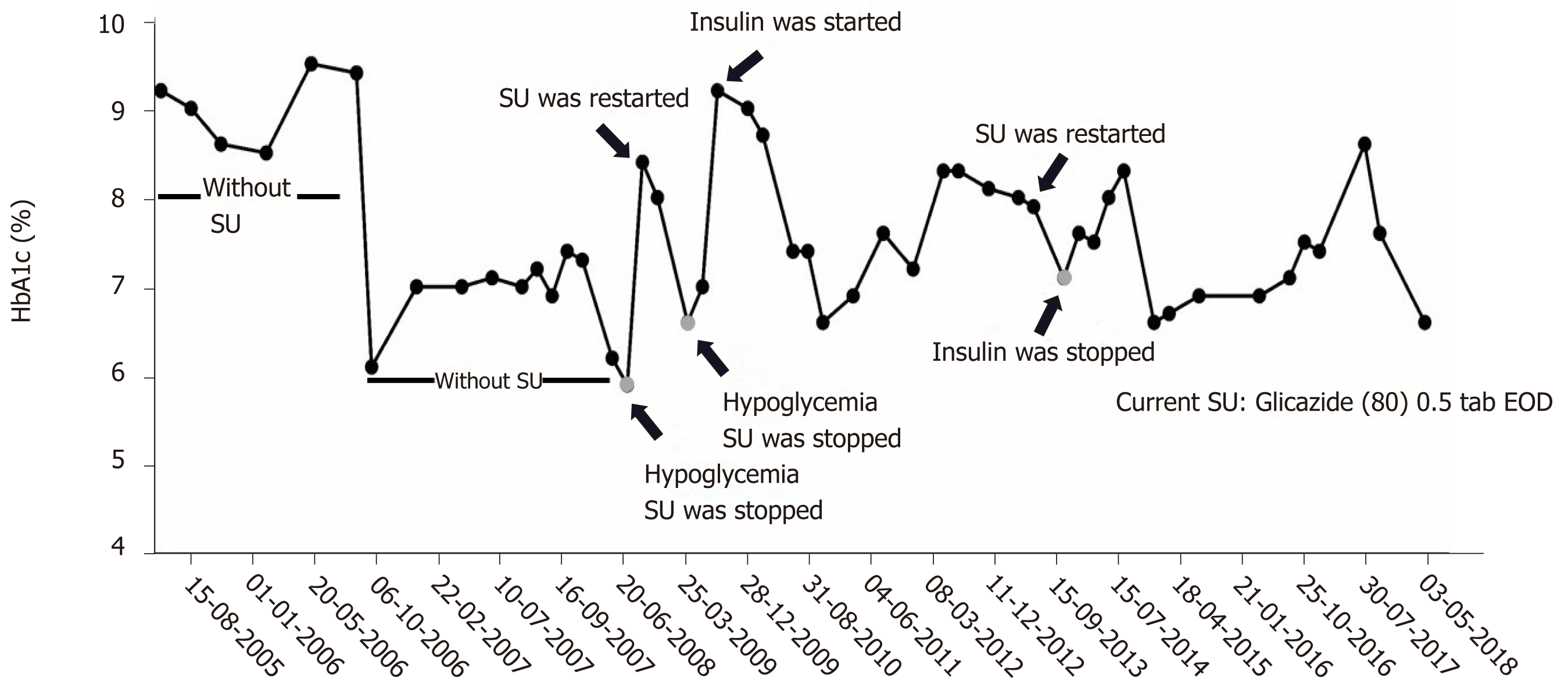
Sanger sequencing of her DNA revealed heterozygous mutation of HNF1A at codon 203 in exon 3 that caused substitution of cysteine for arginine (R203C) (Figure 1). This mutation was also identified in all diabetic family members, but not in non-diabetic family members whose DNA were available for sequencing (Figure 2). The patient’s glycemic control profile (with and without SU) is shown in Figure 3. The results of our analysis revealed that excellent glycemic control could only be achieved when our patient was taking SU. Interestingly – when SU treatment was withdrawn, severe hyperglycemia eventually developed, even when insulin was given. The optimal dose of glicazide in this case was 20 mg per day. This patient continues to do well with no observed diabetic complications.
SU hyperresponsiveness in MODY subtype 3 due to HNF1A mutation.
The patient has been successfully treated with glicazide 20 mg/d, metformin 2000 mg/d and sitagliptin 100 mg/d.
The patient’s glycemic control has been excellence and without hypoglycemic episodes during the last 4 years of follow up. No diabetic complications have deve-loped.
MODY3 is one of the best examples of precision medicine in diabetes. Studies in animal models showed that total deletion of HNF1A resulted in decreased SU uptake by hepatocytes and decreased excretion[2,12,15,16]. Clinical studies in humans demon-strated that MODY3 patients treated with SU exhibited excellent glycemic control, and withdrawal of SU led to severe hyperglycemia – even with insulin treatment. However, dosage adjustment is essential since inappropriate SU dose can lead to hypoglycemia[11]. The current recommendation for treatment of MODY3 patients is to use a very low dose of SU. Caution should be exercised if SU is to be withdrawn from the treatment plan since a deterioration in the patient’s glycemic status can be anticipated. Our MODY3 patient exhibited exceptional plasma glucose control using a very low dose of glicazide, and severe hyperglycemia developed after glicazide was discontinued, even though she was treated with metformin, sitagliptin, and insulin glargine. Moreover, her glicazide dosage was titrated to 20 mg/d to avoid hypogly-cemia, even though the usual dose is up to 80 mg/d for treatment of type 2 diabetes. Upon reaching her maintenance dosage and after stabilization of her blood sugar, insulin therapy could be discontinued and the durability of glycemic control has been almost 4 years (Figure 3). To our knowledge, this is the first report of exceptional response to treatment with SU in Thai MODY3. Our findings are in agreement with those from previous reports in MODY3 patients from different ethnicities, including Caucasian, Saudi Arabian, and Tunisian[17-19]. A study from the United Kingdom reported lower HbA1c and lower BMI at genetic diagnosis, and shorter duration of diabetes to be factors that significantly influence treatment success after treatment with SU in MODY3 patients[20]. However, this finding has not yet been investigated or confirmed in Asian population due to the relatively lower prevalence of MODY3 in this ethnicity.
In this report, we presented and described a 31-year-old Thai MODY3 patient with a heterozygous mutation of HNF1A at R203C who demonstrated excellent diabetic control with a very low dose of SU. To our knowledge, this is the first report of exceptional response to treatment with SU in Thai MODY3. Our findings emphasize the critical role of correct genetic diagnosis, especially in patients with early-onset diabetes.
The authors gratefully acknowledge the patients and family members that generously agreed to participate in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Avtanski D, Biswas SK, Hosseinpour-Niazi S, Hussain SAR, Surani S, Vargas FR S-Editor: Ji FF L-Editor: A E-Editor: Wang J
| 1. | Bansal V, Gassenhuber J, Phillips T, Oliveira G, Harbaugh R, Villarasa N, Topol EJ, Seufferlein T, Boehm BO. Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals. BMC Med. 2017;15:213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345:971-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 775] [Cited by in F6Publishing: 660] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 3. | Siddiqui K, Musambil M, Nazir N. Maturity onset diabetes of the young (MODY)--history, first case reports and recent advances. Gene. 2015;555:66-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017;60:769-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 5. | Tonooka N, Tomura H, Takahashi Y, Onigata K, Kikuchi N, Horikawa Y, Mori M, Takeda J. High frequency of mutations in the HNF-1alpha gene in non-obese patients with diabetes of youth in Japanese and identification of a case of digenic inheritance. Diabetologia. 2002;45:1709-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Hwang JS, Shin CH, Yang SW, Jung SY, Huh N. Genetic and clinical characteristics of Korean maturity-onset diabetes of the young (MODY) patients. Diabetes Res Clin Pract. 2006;74:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Shim YJ, Kim JE, Hwang SK, Choi BS, Choi BH, Cho EM, Jang KM, Ko CW. Identification of Candidate Gene Variants in Korean MODY Families by Whole-Exome Sequencing. Horm Res Paediatr. 2015;83:242-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Ng MC, Cockburn BN, Lindner TH, Yeung VT, Chow CC, So WY, Li JK, Lo YM, Lee ZS, Cockram CS, Critchley JA, Bell GI, Chan JC. Molecular genetics of diabetes mellitus in Chinese subjects: identification of mutations in glucokinase and hepatocyte nuclear factor-1alpha genes in patients with early-onset type 2 diabetes mellitus/MODY. Diabet Med. 1999;16:956-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Lee HJ, Ahn CW, Kim SJ, Song YD, Lim SK, Kim KR, Lee HC, Huh KB. Mutation in hepatocyte nuclear factor-1alpha is not a common cause of MODY and early-onset type 2 diabetes in Korea. Acta Diabetol. 2001;38:123-127. [PubMed] [Cited in This Article: ] |
| 10. | Chèvre JC, Hani EH, Boutin P, Vaxillaire M, Blanché H, Vionnet N, Pardini VC, Timsit J, Larger E, Charpentier G, Beckers D, Maes M, Bellanné-Chantelot C, Velho G, Froguel P. Mutation screening in 18 Caucasian families suggest the existence of other MODY genes. Diabetologia. 1998;41:1017-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Czyrski A, Resztak M, Hermann T. Determination of gliclazide minimum concentration in type 2 diabetes mellitus patients. Biomed Pharmacother. 2018;106:1267-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Sujjitjoon J, Jungtrakoon P, Boonyasrisawat W, Chongjaroen N, Chukijrungroat T, Kooptiwut S, Plengvidhya N, Banchuin N, Yenchitsomanus P. Molecular genetics of monogenetic beta-cell diabetes. Thai J Genetics. 2008;1:93-108. [Cited in This Article: ] |
| 13. | Plengvidhya N, Boonyasrisawat W, Chongjaroen N, Jungtrakoon P, Sriussadaporn S, Vannaseang S, Banchuin N, Yenchitsomanus PT. Mutations of maturity-onset diabetes of the young (MODY) genes in Thais with early-onset type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2009;70:847-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013;34:669-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Kura RR, Kilari EK, Shaik M. Influence of aprepitant on the pharmacodynamics and pharmacokinetics of gliclazide in rats and rabbits. PeerJ. 2018;6:e4798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Fajans SS, Conn JW. Tolbutamide-induced improvement in carbohydrate tolerance of young people with mild diabetes mellitus. Diabetes. 1960;9:83-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Pearson ER, Liddell WG, Shepherd M, Corrall RJ, Hattersley AT. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med. 2000;17:543-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 198] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Khelifa SB, Dendana A, Barboura I, Khochtali I, Chahed H, Ferchichi S, Miled A. Successful switch from insulin to oral sulfonylurea therapy in HNF1A-MODY Tunisian patient with the P291fsinsC mutation. Diabetes Res Clin Pract. 2016;115:133-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Habeb AM, George ET, Mathew V, Hattersley AL. Response to oral gliclazide in a pre-pubertal child with hepatic nuclear factor-1 alpha maturity onset diabetes of the young. Ann Saudi Med. 2011;31:190-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Shepherd MH, Shields BM, Hudson M, Pearson ER, Hyde C, Ellard S, Hattersley AT, Patel KA; UNITED study. A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin. Diabetologia. 2018;61:2520-2527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |