Copyright
©The Author(s) 2023.
World J Diabetes. Feb 15, 2023; 14(2): 76-91
Published online Feb 15, 2023. doi: 10.4239/wjd.v14.i2.76
Published online Feb 15, 2023. doi: 10.4239/wjd.v14.i2.76
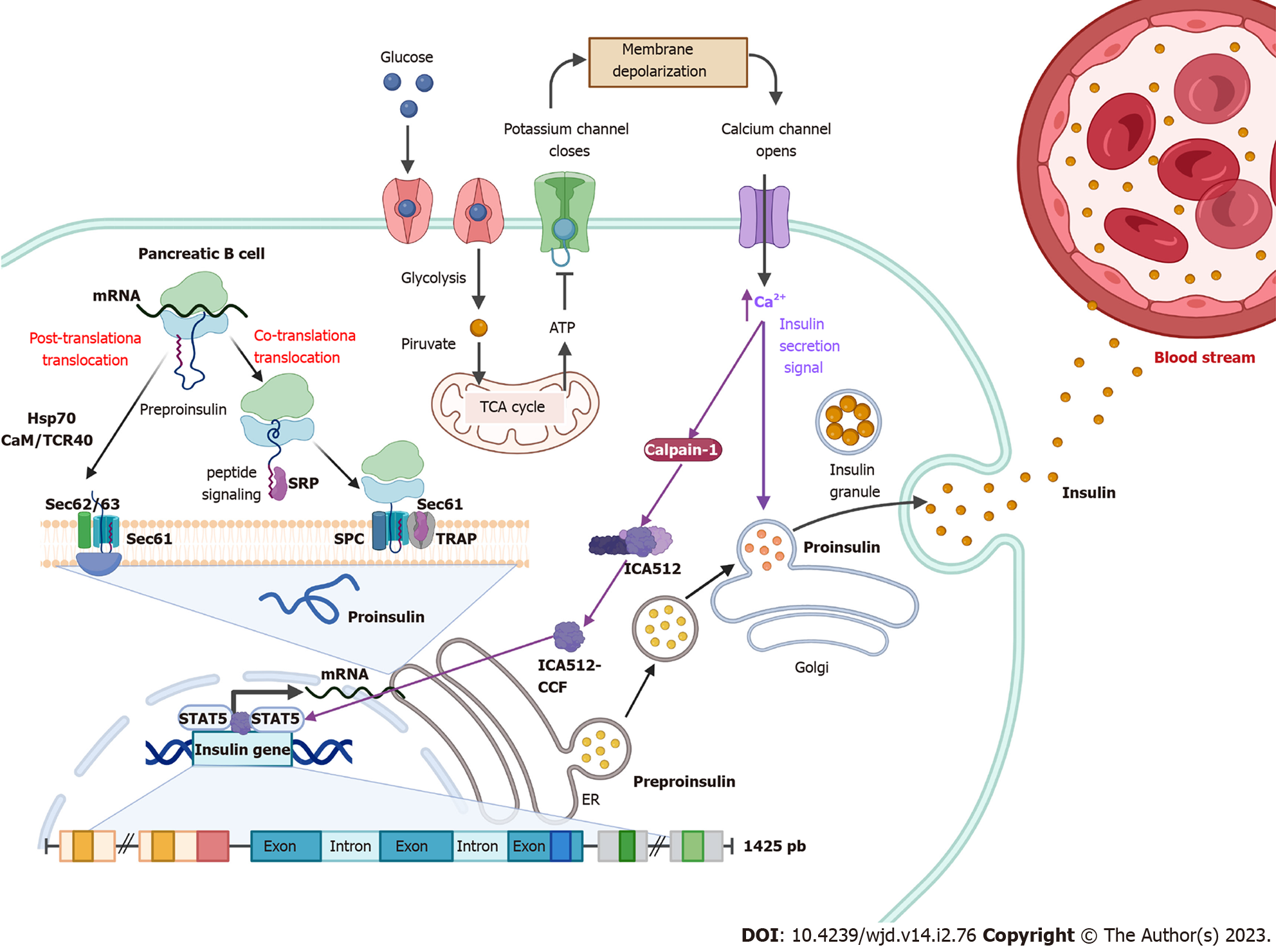
Figure 1 Insulin synthesis.
Following food intake, glucose is internalized into pancreatic β cells and its degradation through glycolysis and the tricarboxylic acid cycle is initiated. Intracellular ATP levels increase, which generates the closure of K+ channels, causing a change in membrane permeability opening Ca2+ channels. Elevated Ca2+ intracellular levels activate calpain-1, a protease that cleaves a cytosolic fragment of islet cell autoantigen 512. The free fragment islet cell autoantigen 512 targets the nucleus and binds to signal transducer and activator of transcription 5, which in turn promotes increased transcription of the insulin gene to mRNA. In the cytosol, insulin is translated as pre-proinsulin that includes a nuclear transport signal peptide that guides pre-proinsulin to the rough endoplasmic reticulum (ER) membrane for translocation to the ER cisternae via two mechanisms: a signal recognition particle-dependent cotranslational translocation; and a signal recognition particle-independent post-translational translocation mechanism. Pro-insulin is generated and folds and stabilizes in its three-dimensional configuration. After acquiring three-dimensional folding, pro-insulin is transferred from the ER to the Golgi via vesicles where pro-insulin is converted to insulin. Also, elevated Ca2+ intracellular levels induce the remodeling of the cytoskeleton and the translocation of insulin granules to the plasma membrane to be subsequently secreted to the blood stream.
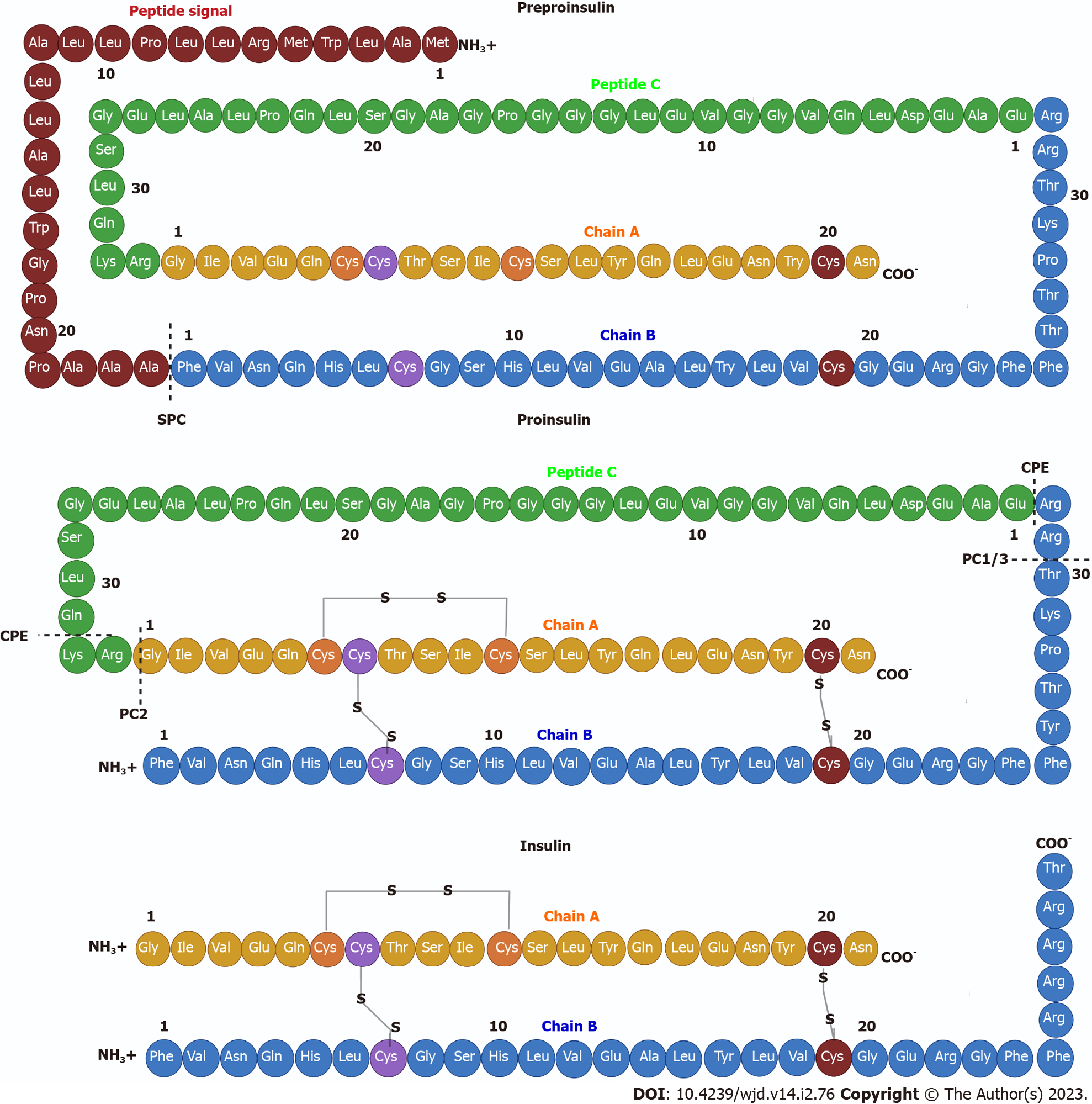
Figure 2 Insulin structure.
Pre-proinsulin is secreted as a polypeptide chain of 110 amino acids (aa), composed of a signal peptide (24 aa), chain A (21 aa), peptide C (33 aa) and a chain B (32 aa). The signal peptide is cleaved by signal peptidase generating pro-insulin, a chain of 86 aa that folds and stabilizes in its three-dimensional configuration by three disulfide bonds between both chains: CysA7-CysB7; CysA20-CysB19; and CysA7-CysA11. Finally, two endoproteases, prohormone convertase 2 and prohormone convertase 1/3, hydrolyze between the basic aa Arg33-Gly1 at the C-peptide and A-chain junction and between the dipeptide Thr30-Arg31 at the B-chain and C-peptide junction, respectively. Subsequently, carboxypeptidase E hydrolyzes between the Gln31-Lys32 aa as well as between Arg32 and Glu1 basic C-termini of the resulting peptide chains, producing a mature insulin protein of 51 aa.
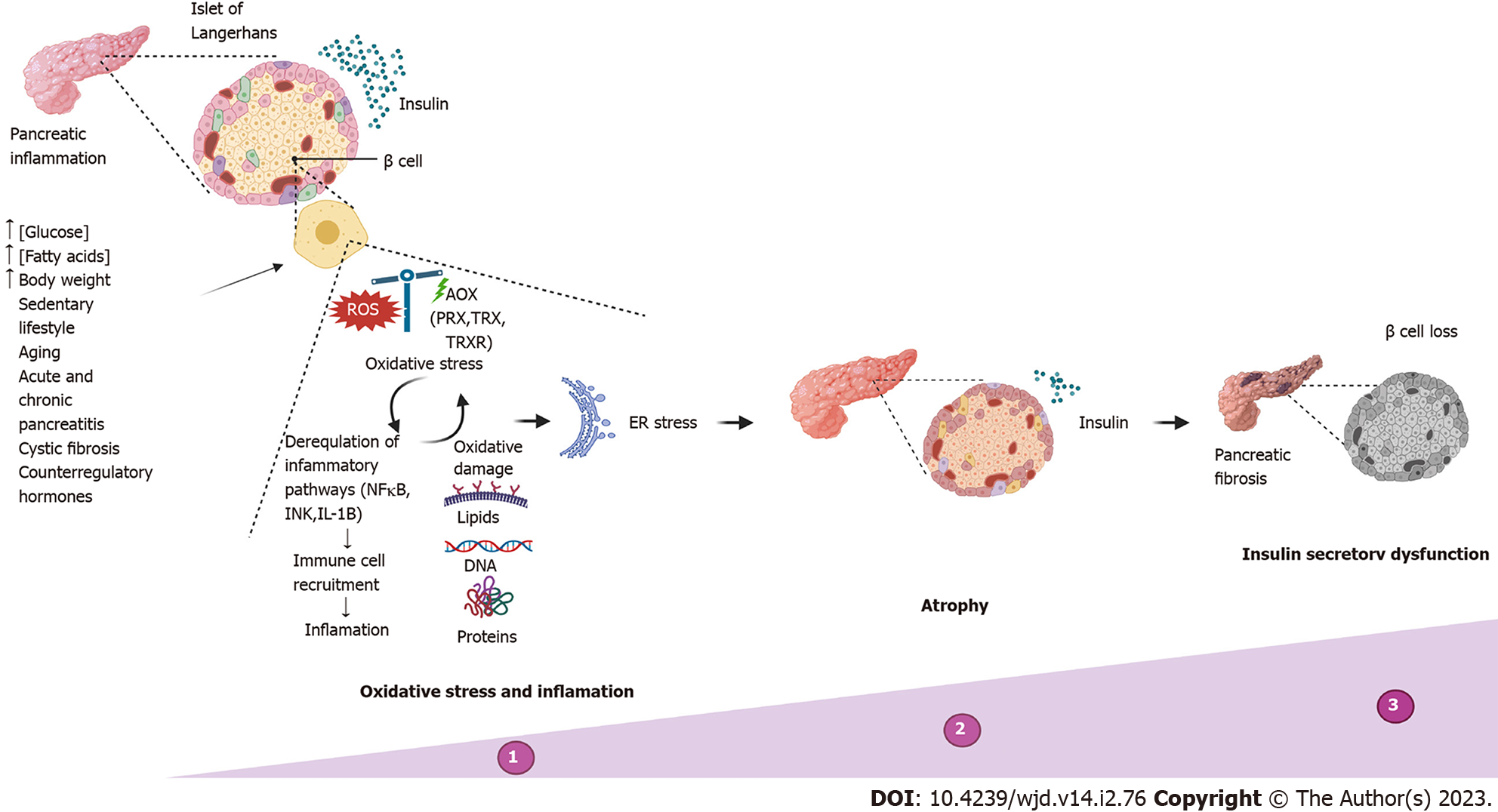
Figure 3 Insulin secretory dysfunction.
Timeline of abnormalities in insulin secretion due to the most common causes, reflecting progressive deterioration in functional β cell mass. ER: Endoplasmic reticulum.
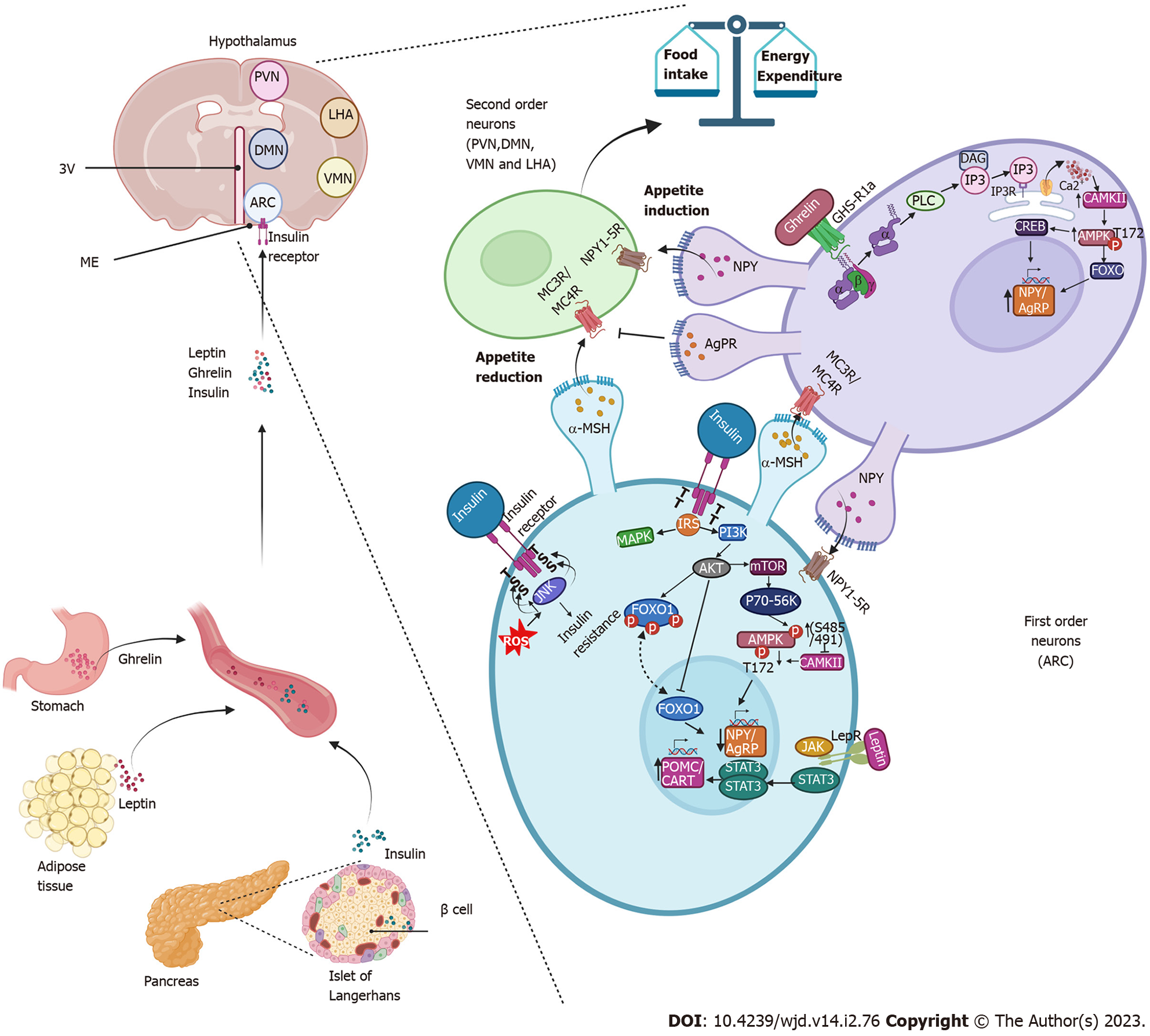
Figure 4 Insulin promotes decreased appetite in the arcuate nucleus.
Insulin is secreted by the cells of the pancreas and through the circulation reaches the arcuate nucleus of the hypothalamus. It binds to its receptor on first-order neurons, triggering the phosphatidylinositol-3-kinase/protein kinase B signaling pathways and forkhead box protein O1 repression, resulting in decreased expression of neuropeptide Y (NPY) and agouti-related protein resulting in an anorexigenic effect. Like insulin, leptin activates anorexigenic signaling pathways by binding to its receptor, which activates the Janus tyrosine kinase/signal transducer and activator of transcription pathway, promoting the expression of the anorexigenic peptide precursor neuropeptide of α-melanocyte-stimulating hormone and transcript regulated by cocaine and amphetamines and with it the release of the α-melanocyte-stimulating hormone that activates the melanocortin receptors (MC3R/MC4R) in the neurons of the second order. Together, insulin and leptin signals amplify the anorexigenic effect. During fasting periods, ghrelin activates the growth hormone receptor 1a and promotes the activation of the adenosine monophosphate-activated protein kinase pathway that promotes the expression of NPY/agouti-related protein, stimulates orexigenic receptor Gi-coupled NPY in second-order neurons and prevents α-melanocyte-stimulating hormone from binding to melanocortin receptors (MC3R/MC4R), driving the orexigenic signal. 3V: Third ventricle; DAG: Diacylglycerol; IP3: Inositol triphosphate; IP3R: Inositol triphosphate receptor; ME: Median eminence; PLC: Phospholipase C; ROS: Reactive oxygen species.
- Citation: De la Cruz-Concepción B, Flores-Cortez YA, Barragán-Bonilla MI, Mendoza-Bello JM, Espinoza-Rojo M. Insulin: A connection between pancreatic β cells and the hypothalamus. World J Diabetes 2023; 14(2): 76-91
- URL: https://www.wjgnet.com/1948-9358/full/v14/i2/76.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i2.76