Published online Sep 15, 2014. doi: 10.4251/wjgo.v6.i9.311
Revised: November 7, 2013
Accepted: December 9, 2013
Published online: September 15, 2014
Processing time: 394 Days and 11.5 Hours
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a noninvasive epithelial neoplasm of mucin-producing cells arising in the main duct (MD) and/or branch ducts (BD) of the pancreas. Involved ducts are dilated and filled with neoplastic papillae and mucus in variable intensity. IPMN lacks ovarian-type stroma, unlike mucinous cystic neoplasm, and is defined as a grossly visible entity (≥ 5 mm), unlike pancreatic intraepithelial neoplasm. With the use of high-resolution imaging techniques, very small IPMNs are increasingly being identified. Most IPMNs are solitary and located in the pancreatic head, although 20%-40% are multifocal. Macroscopic classification in MD type, BD type and mixed or combined type reflects biological differences with important prognostic and preoperative clinical management implications. Based on cytoarchitectural atypia, IPMN is classified into low-grade, intermediate-grade and high-grade dysplasia. Based on histological features and mucin (MUC) immunophenotype, IPMNs are classified into gastric, intestinal, pancreatobiliary and oncocytic types. These different phenotypes can be observed together, with the IPMN classified according to the predominant type. Two pathways have been suggested: gastric phenotype corresponds to less aggressive uncommitted cells (MUC1 -, MUC2 -, MUC5AC +, MUC6 +) with the capacity to evolve to intestinal phenotype (intestinal pathway) (MUC1 -, MUC2 +, MUC5AC +, MUC6 - or weak +) or pancreatobiliary /oncocytic phenotypes (pyloropancreatic pathway) (MUC1 +, MUC 2-, MUC5AC +, MUC 6 +) becoming more aggressive. Prognosis of IPMN is excellent but critically worsens when invasive carcinoma arises (about 40% of IPMNs), except in some cases of minimal invasion. The clinical challenge is to establish which IPMNs should be removed because of their higher risk of developing invasive cancer. Once resected, they must be extensively sampled or, much better, submitted in its entirety for microscopic study to completely rule out associated invasive carcinoma.
Core tip: The authors review the main pathological features of intraductal papillary mucinous neoplasm (IPMN) of the pancreas, including diagnostic criteria and relevance of macroscopic (i.e., main duct, branch duct and mixed or combined) and microscopic (i.e., gastric, intestinal, pancreatobiliary and oncocytic) IPMN classification. Different pathways, mucin immunophenotypes and invasive carcinoma related to IPMN are addressed. Differential diagnosis with pancreatic intraepithelial neoplasm, mucinous cystic neoplasm and other mucinous and non-mucinous pancreatic cystic lesions are also included.
- Citation: Castellano-Megías VM, Andrés CID, López-Alonso G, Colina-Ruizdelgado F. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol 2014; 6(9): 311-324
- URL: https://www.wjgnet.com/1948-5204/full/v6/i9/311.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i9.311
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a grossly visible, noninvasive epithelial neoplasm of mucin producing cells arising in the main pancreatic duct or its branches. Intraductal growth of neoplastic cells usually forms papillae in a variable extension, although it can rarely be completely flat. Involved ducts are dilated and filled with mucus in variable intensity[1-4]. The mucus produced by the IPMN can protrude through the duodenal papilla and this sign, so-called “fish-eye ampulla”, is virtually diagnostic, although it has been observed in only about 25% of cases[5,6]. IPMN lacks ovarian-type stroma, unlike mucinous cystic neoplasm (MCN) of the pancreas[7]. IPMN is defined as a grossly visible entity, unlike pancreatic intraepithelial neoplasm (PanIN), which is defined as a microscopic lesion[8].
IPMN was previously reported under a variety of terms (mucinous producing cancer[9], ductectatic-type mucinous cystadenoma and cystadenocarcinoma[10], diffuse villous adenoma[11] and intraductal papillary neoplasm of the pancreas[12]) that referred to some of the main features of these lesions. Currently, use of these terms is discouraged. Intraepithelial papillary lesions morphologically analogous to IPMNs develop in the biliary tree, including bile ducts[13], gallbladder[14] and ampullary region[15], and are also reported under a variety of terms.
Most IPMNs are diagnosed between 60 and 70 years of age. There is a slightly higher prevalence in men than women[16]. IPMNs are mostly located in the pancreatic head (70%). About 20% are placed in the body-tail and about 5%-10% show diffuse involvement of the gland. Most are solitary lesions but 20%-40% are multifocal[1,17]. IPMNs can reach a large size before diagnosis because of the slow and indolent growth. However, with the use of high-resolution imaging techniques, very small incidental pancreatic cysts, including IPMNs, are increasingly being identified[18,19]. In the Laffan et al[19] series, radiological incidental pancreatic cysts were detected with a mean size of 8.9 mm (range 2-38 mm) in 2.6% of adults without known pancreatic disease. It has been suggested that most of them are IPMNs originating from the small branch ducts but pathological data are missing[20]. On the other side, older surgical data, in which IPMN represents 20% of all pancreatic cysts, probably underestimate its prevalence[1].
IPMNs may exhibit different degrees of dysplasia in the epithelium but even those with high-grade dysplasia or carcinoma in situ have a very good prognosis after resection[21-24]. However, about 40% of IPMNs show invasive pancreatic carcinoma at diagnosis[18]. The prognosis of these patients critically worsens when invasive carcinoma arises, although in the case of minimal invasion, the prognosis is not as severe[23,25]. The clinical challenge is to establish which IPMNs can be managed by clinical and radiological follow-up without requiring surgical excision and which should be removed because they are likely to develop invasive cancer. Progress has been made in the preoperative assessment of the risk of malignancy of pancreatic cystic lesions. Currently, there is an international consensus for the preoperative management of these patients based on clinical and radiological criteria, published in 2006 (the so called Sendai criteria) and updated in 2012 (the Fufuoka guidelines)[7,18]. Nevertheless, the preoperative diagnostic accuracy of pancreatic cystic neoplasms is still far from optimal[26,27].
IPMN appears as a dilatation of the main duct or as one or more cysts communicated with the excretory duct system. IPMNs are cystic lesions so the observation of any solid nodule should be suspected of associated invasive carcinoma. However, it should be noted that the invasive carcinoma, especially if small, may be overlooked macroscopically.
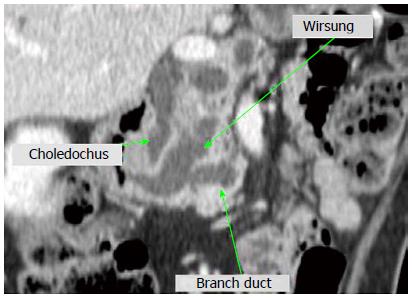
IPMN is defined as a grossly visible lesion and, mostly based on radiological criteria, it is typically considered to be 1 cm or more in size[1,7] (Figure 1). More recently, the Fukuoka guidelines have proposed to reduce the minimum size for radiological diagnosis of IPMN to 5 mm, which increases diagnostic sensitivity without losing specificity. According to this consensus, pancreatic cysts of > 5 mm in diameter that communicate with the main pancreatic duct, especially if there is no pancreatitis, and/or diffuse dilation of the main pancreatic duct of > 5 mm without other causes of obstruction are sufficient radiological criteria for IPMN[18].
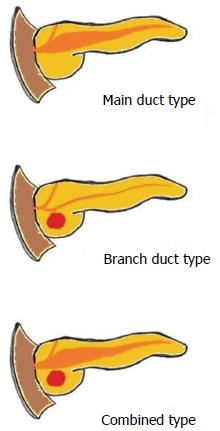
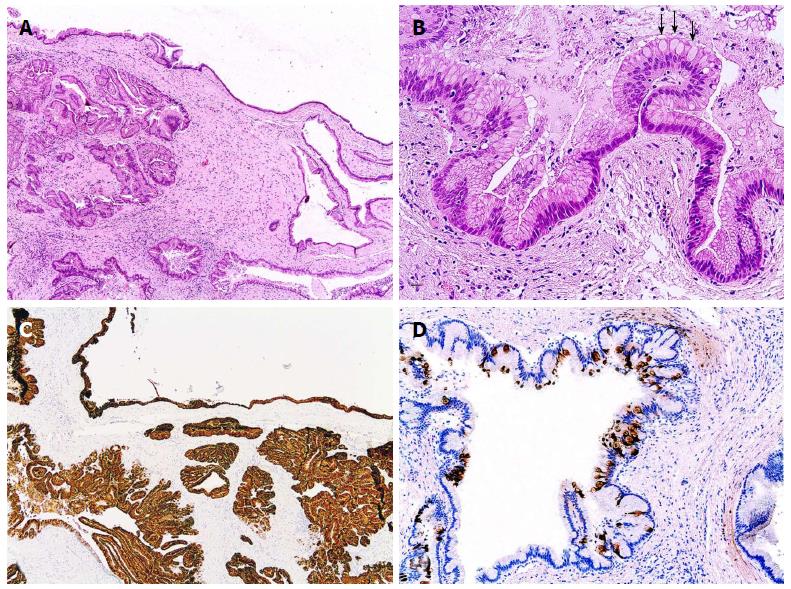
According to their main location, IPMNs are classified into main duct (MD) type (16% to 36%), branch duct (BD) type (40% to 65%) and mixed or combined type (15% to 23%)[21,23,28,29] (Figure 2). This classification reflects biological differences with important prognostic implications[23,30]. Most MD type and combined type IPMNs exhibit malignancy (i.e., high-grade dysplasia or carcinoma), with about 45% having an associated invasive carcinoma[18,22,31]. In contrast, most BD type show low-grade dysplasia and only about 15% are associated with invasive carcinoma[18]. The natural history of BD type under 30 mm in size and without mural nodules is particularly favorable[5,32]. Currently, this macroscopic classification has a substantial practical impact on the preoperative clinical management based on imaging findings[7,18].
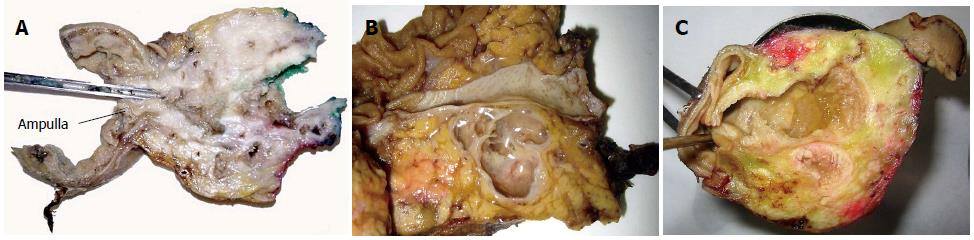
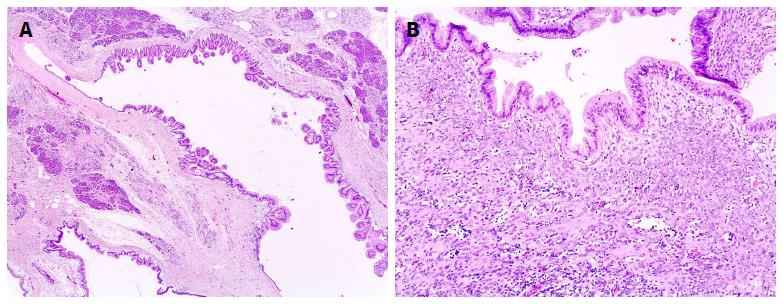
MD type (Figure 3A) is essentially located in the main pancreatic duct[11,18]. At external examination, the pancreas may be thickened in the affected area. After opening, this type typically shows dilatation of the main duct with irregular outline and the lumen is filled by mucus and villous or papillary projections. The rest of the pancreas often shows the appearance of obstructive chronic pancreatitis due to pancreatic duct obstruction[17]. Most of the MD type are located in the pancreatic head but one third of them are in the body and tail, and almost 5% in the entire main pancreatic conduct (diffuse MD type)[22]. Eventually, some cases of MD type are multifocal. However, a particular lesion may be macroscopically from a focal type but microscopically exhibit multifocal or diffuse extension throughout the duct.
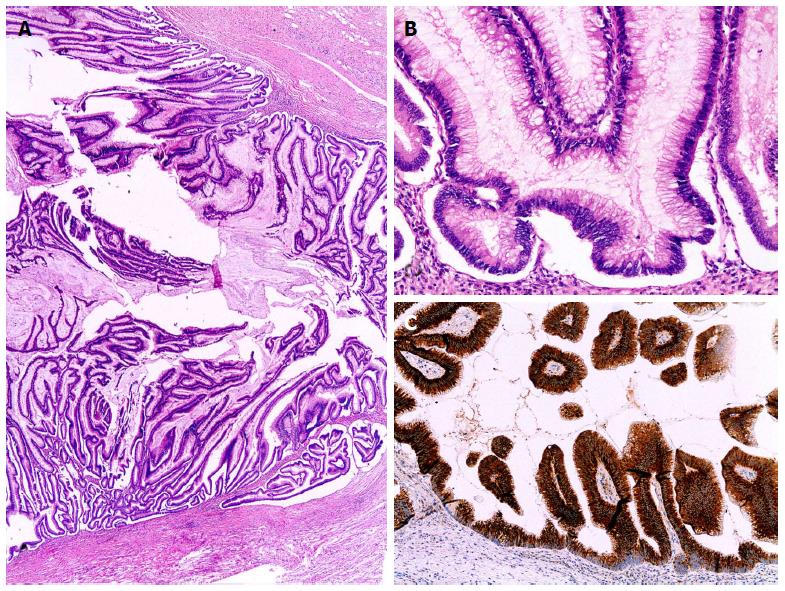
BD type (Figure 3B) is located predominantly in secondary branches of the pancreatic ductal system[1,18]. Typically, the affected duct has the appearance of a mucus-filled cyst. As there is no main pancreatic duct obstruction, the remaining pancreas may have normal appearance[17,33]. Most of the IPMN of BD type occurs in the pancreatic head and very commonly in the uncinate process. About 25%-40% are multifocal[18,22].
The mixed or combined type of IPMN (Figure 3C) primarily affects both the main pancreatic duct and secondary branches[1,18]. Hypothetically, combined type of IPMN might result from the progression of MD or BD types or it could be a distinct disease. Clinical and biological characteristics are similar to those of the MD type, so it is thought that combined IPMN is most likely an extension of the MD type to the branch ducts[22,30].
Uncommonly, the neoplastic papillae extends out of the ampulla and onto the surface of the periampullar duodenum or into the distal common bile duct[17].
Also infrequently, IPMN can develop a fistula to neighboring organs, among them the duodenum, stomach, choledochus, colon and small intestine. The fistula may be related to benign IPMNs (i.e., low or moderate degree of dysplasia), malignant IPMNs (i.e., high-grade dysplasia) or invasive carcinoma associated with IPMN (often a colloid carcinoma)[34]. Two scenarios can be observed in the pathogenesis of these fistulas: (1) mechanical penetration due to excessive pressure in the mucin filled ducts, in addition to inflammatory stimulation or autodigestion of enzyme rich fluids; and (2) direct invasion, i.e., with presence of invasion of the tissue around the fistula[34,35]. Mechanical penetration can occur regardless of the presence of malignant cells at the surface of the fistula (without direct tissue invasion by carcinoma). In conclusion, the presence of fistulas in IPMN does not necessarily mean malignancy and should not be confused with invasive carcinoma.
In rare cases, IPMN has been described as causing pseudomyxoma peritonei[36], for instance by associated acute pancreatitis with fistula formation to the abdominal cavity[37] or after intraoperative manipulation of the pancreas[22].
Extensive pancreatic calcification has rarely been described in patients with IPMN. This obstructive calcifying pancreatitis, presumed to be caused by the IPMN, may lead to preoperative diagnostic confusion and delay in the diagnosis of the papillary neoplasia[38,39].
Histologically, IPMN is a heterogeneous group of lesions with different degrees of dysplasia and different cellular phenotypes. The underlying stroma shows a conventional fibrous tissue, which by definition can not be of ovarian type, as seen in MCN[1].
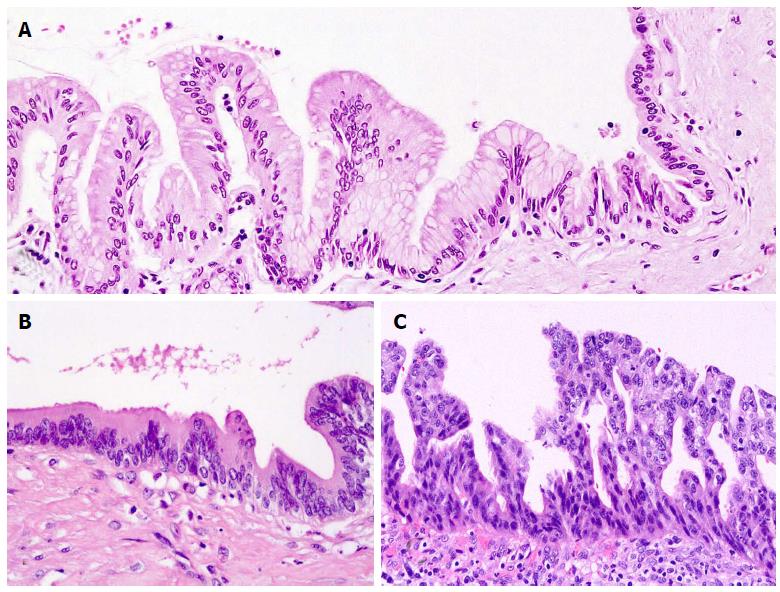
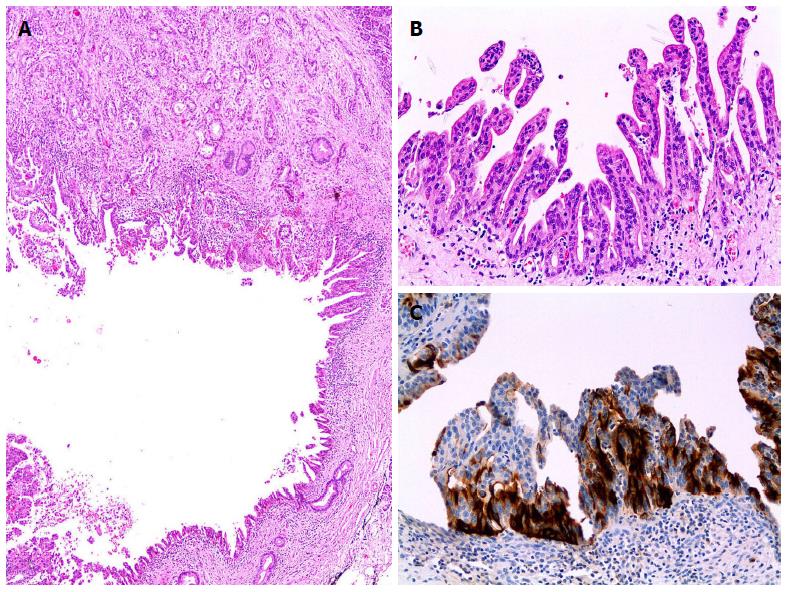
Based on the degree of cytological atypia and abnormal crowding of the epithelium, IPMN is classified into three categories: IPMN with low-grade dysplasia, IPMN with intermediate-grade dysplasia and IPMN with high-grade dysplasia (Figure 4). This nomenclature, currently adopted by the WHO system, replaces the terms of adenoma (low grade), borderline (intermediate grade) and carcinoma in situ (high grade dysplasia)[1,7]. Low-grade dysplasia is characterized by a uniform monolayer of columnar cells with basal nuclei showing no or minimal atypia. In the intermediate-grade of dysplasia, nuclear atypia is higher, with nuclear pleomorphism, nuclear enlargement and pseudostratification. In high-grade dysplasia, there is marked cytological atypia and complex architecture with cribriform groups and budding of neoplastic cells into the lumen[4,17]. It is common to observe different grades of dysplasia within a given lesion, which suggests the development of dysplasia from a lower to a higher grade. The distinction of dysplasia grade is important, with associated invasive carcinoma commonly immersed in areas of high-grade dysplasia[40,41]. In each individual case, the lesion should be classified according to the highest grade of dysplasia observed[1].
On the basis of the cytoarchitectural features and immunophenotype, IPMNs are classified into four histopathological types: gastric, intestinal, pancreatobiliary and oncocytic IPMNs[1], accounting for 49%-63%, 18%-36%, 7%-18% and 1%-8% of total cases in two large series[3,42] (Table 1). This classification is not only descriptive but also indicative of different pathways of differentiation and progression to invasive carcinoma[23,43-48]. The above nomenclature prevails over other terms proposed for these lesions[3]. The so called villous dark cell, papillary clear cell and compact cell types respectively correspond to intestinal, gastric and oncocytic cell types[49,50], whereas null cell type corresponds to gastric cell type[51].
Core proteins for mucins (MUCs) can be detected by immunohistochemistry. The mucin expression profile by the IPMN cells is a major contributor to their phenotypic classification. Mucins are high molecular weight glycoproteins produced by different types of epithelial cells. Some mucins are normally located in the cell membrane, like MUC1 (also called mammary-type mucin or pan-epithelial membrane mucin), whereas others are normally secretory products, including MUC2 (intestinal type gel forming mucin), MUC5AC (gastric surface mucous epithelial mucin) and MUC6 (gastric pyloric glandular mucin)[52,53].
In normal pancreatic tissue, there is MUC1 expression (limited to centroacinar cells, intercalated and intralobular ducts and focally in the interlobular ducts) and sometimes there is expression of MUC6 (limited to the acini) but MUC2 and MUC5AC are not expressed[50]. In pancreatic neoplasms, MUC1 is considered a marker of aggressiveness, being expressed in some IPMNs, higher-grade cases of PanIN and in conventional (i.e., tubular) ductal adenocarcinoma. On the contrary, MUC2 is considered a marker of a more indolent phenotype, being expressed in some IPMNs and in colloid carcinoma[43].
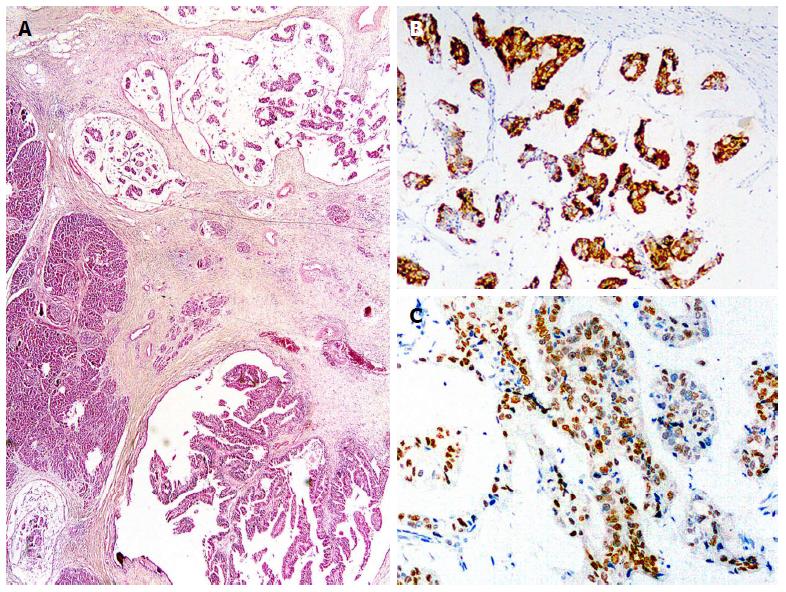
Gastric type IPMN is frequently observed in BD type. The great majority of gastric IPMNs exhibit only low-grade dysplasia and association with invasive carcinoma is uncommon. It has been observed that, when developing, invasive carcinomas are conventional type with more aggressive characteristics and with a poorer prognosis than those arising from intestinal or pancreatobiliary IPMNs[23,16]. Because the gastric type IPMNs associated with these invasive carcinomas is usually benign (i.e., with only low or intermediate grade of dysplasia), it has been questioned whether in these cases the gastric type IPMN represents the invasive carcinoma or whether it is just its background or coexisting benign IPMN[42]. Gastric type IPMN consists of columnar cells with basal nuclei and abundant apical cytoplasmic mucin, resembling the foveolar gastric epithelium (it is also called gastric foveolar type IPMN). These lesions are often mainly flat or with low papillary pattern, consisting in thick finger-like papillae[3]. Immunoprofile consists of diffuse expression of MUC5AC and MUC6 without expression of MUC1 and MUC2, although scattered MUC2 positive goblet cells can be present into the lesion[16,44,48,50]. Gastric mucins may show a distribution which mimics mucins found in the gastric mucosa, namely, increased expression of MUC5AC in the superficial or papillary areas, simultaneously of MUC6 located in the basal areas[50] (Figure 5).
Intestinal type IPMNs mimics villous adenomas of the colon. They form elongated papillae of columnar cells with enlarged cigar-like nuclei[3]. Diffuse expression of MUC2 and MUC5AC, weak or negative expression of MUC6 and negativity for MUC1 is the mucin immunoprofile of this type[16,50,48]. In addition, intestinal IPMN exhibits diffuse expression for CDX2, a transcriptional factor related to intestinal differentiation that, like MUC2, has tumor suppressor activity[54]. The intestinal type IPMN frequently exhibits an intermediate or high-grade of dysplasia. It occurs more frequently in the main duct and, when associated with invasive carcinoma, this is often a colloid adenocarcinoma[23,51]. In the absence of invasive carcinoma, intestinal IPMN seems to have a greater potential for long-term recurrence than non-invasive IPMN of other types. Recurrence in the remnant pancreas may be due to multifocality not recognized at the time of surgery or to metachronous development[23] (Figure 6).
Pancreatobiliary type IPMNs usually show high-grade dysplasia and is likely to have a strong predisposition to develop invasive carcinoma[23]. Associated invasive carcinomas usually are of conventional type[23,51]. Pancreatobiliary type IPMN consists of more cuboidal cells with rounded nuclei, often with prominent nucleoli. The neoplastic cells are organized into thin complex and branching papillae with bridging and cribiform patterns[3]. The neoplastic cells express MUC1 and MUC5AC, sometimes MUC6, and are negative for MUC2[3,48] (Figure 7).
Oncocytic type IPMNs (also known as intraductal oncocytic papillary neoplasm) are characterized by neoplastic cells with abundant granular eosinophilic cytoplasm (due to the presence of numerous mitochondria) and also intracellular mucin[1,55]. Most of them have high-grade dysplasia, with complex thick papillae and cribriform structures. The neoplastic cells express MUC1 and MUC6. Expression of MUC5AC is controversial, being constantly present according to some authors but limited to scattered goblet cells according to others. The scattered goblet cells also express MUC2[48,56,57]. Invasive carcinoma associated with oncocytic type IPMN often conserves the oncocytic features[23,42]. An association between the oncocytic type IPMN and minimally invasive carcinoma has been observed[23].
In some cases, different phenotypes can be observed in the same lesion. Each lesion should be classified according to the predominant phenotype, although all the present phenotypes should be recorded[3]. The most common coexistences are gastric with intestinal or gastric with pancreatobiliary type[42]. On the contrary, it is very rare to observe intestinal and pancreatobiliary types together[16]. Oncocytic type has been observed to be associated with gastric and pancreatobiliary types[56]. It has been suggested that the gastric phenotype corresponds to less aggressive uncommitted cells with the capacity to evolve to intestinal phenotype (intestinal pathway) or pancreatobiliary/oncocytic phenotypes (pyloropancreatic pathway) becoming more aggressive[44,48,51]. Gastric foveolar epithelium-like cells (also called null cell type cells) similar to cells of the gastric papillary areas of IPMNs can usually be observed lining the nonpapillary cystic areas of different IPMNs[51]. Pancreatic duct glands are blind-ending outpouches of major ducts with a possible role in epithelial renewal and repair. Epithelium of these glands is a specialized compartment with production of gastric type mucin (MUC6+) in its normal state. When chronically injured, it becomes hyperplastic and it acquires de novo expression of MUC5AC. It has been speculated that these pancreatic duct glands are a source of gastric mucinous metaplasia and could be the origin of PanIN[58] and IPMNs[6], in addition to its possible role in regeneration and protection of the major ducts.
Frozen study of pancreatic cut surface during resection of IPMN is accurate to evaluate the completeness of resection. The accuracy of frozen study averages 95%[59]. If invasive carcinoma or high-grade dysplasia is seen in the pancreatic margin, this should be extended. Further resection in cases with lesser degrees of dysplasia in the margin is controversial[18] but may be considered in some cases, depending on the patient’s age and the macroscopic type IPMN among other factors[60,61]. Recurrences after resection of non-invasive IPMN with free margin may occur and can be attributed to multifocality[60].
The distinction between IPMN and PanIN may be almost impossible in some cases, although this distinction is not considered crucial in assessing the margin, with the distinction of the degree of dysplasia being most relevant[18]. Those wishing to obtain a pancreatic margin without any degree of dysplasia should be aware that intraoperative differential diagnosis of low-grade dysplasia vs non dysplastic epithelium with reactive changes can be impossible to achieve. This should be considered to avoid unnecessary or useless resections. The presence of mucus or duct dilatation at the cut surface does not indicate any additional necessity for resection. De-epithelization of the pancreatic duct margin has been observed to be a prognostic factor for recurrence by some authors and thus they have proposed to do additional resection in cases of eroded epithelium[60].
About 40% of IPMNs are associated with invasive pancreatic carcinoma, although the reported risks of malignancy are quite population-dependent and vary considerably (with range between 1.4% and 80.8%)[18,29]. Invasive carcinoma can be uni- or multifocal and occurs most often in MD and combined type than in BD type[18,22]. In patients with IPMN, the distinction must be made between adenocarcinoma derived from IPMN and adenocarcinoma concomitant with IPMN[62]. The first evidently develops from IPMN, while the latter occurs in the pancreas with IPMN but in another location of the organ, therefore without an obvious topological relationship and in the absence of histological transition between the two lesions. Sometimes, the possible relationship between IPMN and invasive carcinoma remains undetermined. In a large series of patients with IPMNs and associated adenocarcinoma, 66%, 17% and 16% corresponded to adenocarcinoma derived from IPMN, concomitant with IPMN, and undetermined, respectively[63]. Among the general population with IPMN, the risk of developing invasive pancreatic carcinoma separately from IPMN was estimated to be 2.8% in a recent cohort analysis[29].
Most invasive carcinomas related to IPMNs are colloid (mucinous noncystic) carcinomas and conventional (tubular) carcinomas. Mixed colloid-tubular carcinomas or adenocarcinomas with focal colloid features also occur[17,23,24,29,63-65]. Colloid carcinoma of the pancreas is very commonly associated with IPMN[51]. In fact, some authors argue that it virtually never exists without an associated IPMN (whose detection would depend on the extent of the tumor sampling)[65]. Most IPMNs related to colloid carcinoma are intestinal type[23]. Like intestinal type of IPMN, colloid carcinoma shows diffuse expression of CDX2 and MUC2 (i.e., features of intestinal differentiation)[51] (Figure 8). Prognosis of colloid carcinoma is considered better than conventional ductal carcinoma[18,66]. Conventional invasive adenocarcinoma related to IPMN is most often associated with pancreatobiliary type IPMN (Figure 7A). Both share the more aggressive immunohistochemical profile consisting of MUC1 expression and lack of MUC2 and CDX2 expression[23,51].
In general, the prognosis of patients with invasive carcinoma associated with IPMN is better than that of patients with ordinary pancreatic adenocarcinoma (i.e., patients without IPMN), but when matched by disease stage, no prognostic differences appear to exist between the groups, except at an early stage. Best overall prognosis of these patients may lie in their greater frequency of early stage cases and a higher prevalence of colloid carcinomas[21,41,67,68]. A lower frequency of other adverse histological features (i.e., vascular invasion, perineural invasion, involvement of surgical margins and poor tumor differentiation) contribute to better prognosis of IPMN-associated invasive carcinomas[69].
Some authors have observed that invasive carcinoma has a better prognosis if depth invasion is limited[23,25,70-72]. This so called minimally invasive carcinoma has been defined as tumor with slight invasion beyond the pancreatic duct wall[23,70,71] or as carcinoma with infiltration depth up to 5 mm[25,72]. Fukuoka guidelines recommend avoiding use of the term “minimally invasive” because of its variable definition. Instead, it is proposed to substage the category T1 into T1a if carcinoma infiltrates up to 0.5 cm, T1b if > 0.5 cm up to 1 cm, and T1c if infiltrates between 1 and 2 cm[18]. Minimal invasion is more frequently observed in intestinal and oncocytic types of IPMNs than in gastric and pancreatobiliary types[23,72]. Frequently, minimally invasive carcinoma related to IPMN is colloid type[63].
Some patients with resected IPMN without associated invasive carcinoma subsequently develop local invasive carcinoma or metastatic lesions. Multifocal disease with synchronous or metachronous development of tumor in the remnant unresected pancreas may explain the origin of some of these recurrences[24]. In other cases, it is possible that a preexisting small focus of invasive carcinoma passed unnoticed in the pathological study. Because of its critical prognostic significance, a major objective for the pathologist is to rule out the presence of invasion. Initial sampling of the surgical specimen should include all nodular areas because of its higher suspicion of malignancy. As invasive carcinoma may be overlooked by gross examination (especially if it is of small size), the tumor must be extensively sampled or, much better, submitted in its entirety for microscopic study. Measuring the size of invasive carcinoma irrespective of the size of IPMN is required for appropriate staging. If multiple invasive foci exist, they must be measured separately, highlighting the size of the largest focus.
There is usually little difficulty in recognizing invasive carcinoma associated with IPMN when tumor cells are observed penetrating the tissue with a classic infiltrative growth pattern. However, like in other mucin-secreting tumors, IPMNs can exhibit tumor growth by duct expansion (expansive growth) as well as mucous rupture or mucin spillage into the stroma, whose interpretation is controversial[25,73]. Lakes of mucin in the stroma may correspond to colloid carcinoma but also may be due to rupture of a mucus filled duct, presumably by the high intraluminal pressure produced by the mucus itself. IPMN desquamated cells could be transported to the stroma by the extruded mucin, completely simulating colloid carcinoma. Acellular mucin extruded into stroma is not considered invasive cancer (Figure 9). In contrast, mucin spillage containing neoplastic cells is better considered invasive carcinoma[1,17]. On another issue, IPMNs should not be confused with the rarest pancreatic adenocarcinomas with cystic papillary pattern, consisting of large caliber malignant glands with intraluminal papillary structures and pools of intraluminal mucin that mimic noninvasive cystic neoplasms. Elastin stains are very helpful for distinction: unlike normal pancreatic ducts and ducts with IPMN that typically are surrounded by a layer of elastin fibers, there are no elastin fibers around these large invasive malignant glands[74].
A major preoperative (i.e., mainly clinical and radiological) differential diagnosis of IPMNs includes neoplastic and non-neoplastic pancreatic cysts: serous cytadenoma (in particular the oligocystic or macrocystic variant)[75], MCNs, solid pseudopapillary neoplasm[76], retention cyst[4], pseudocyst and other less common or clinically relevant entities[20]. In addition, usually solid pancreatic lesions that occasionally are dominated by a cystic, papillary or papillocystic pattern must be considered in this differential diagnosis: acinar cell carcinoma[77], pancreatic endocrine tumors[78] and pancreatic duct adenocarcinoma[74]. In surgical specimens, histological findings usually allow solving the above cited differential diagnosis, sometimes with the assistance of immunohistochemistry. In preoperative management, cyst fluid or pancreatic juice cytology can increase clinical and radiological accuracy diagnoses in pancreatic cysts regarding the distinction between mucinous and non mucinous lineage and malignancy identification. Mucinous cyst cytology cannot accurately discriminate between IPMN and MCN and, although cytology shows high specificity for detecting malignancy, sensitivity is low[79]. Sensitivity for malignancy detection increases if cases with cytological diagnosis of high grade atypia are included, but this reduces the specificity[80,81]. Carcinoembryonic antigen (CEA) pancreatic cyst fluid level may contribute to distinction between mucinous (high CEA levels) and non mucinous (low or no CEA levels), but does not differentiate between benign and malignant. Some authors warn about IPMN dissemination after puncture because of the potential risk of leakage of cyst content. Currently, Fukouka guidelines consider cytological study of mucinous-like cystic lesions in general limited to research, except in centers with expertise in endoscopic ultrasound - fine needle aspiration (EUS-FNA) and cytological interpretation where cytological analysis is recommended for the evaluation of small BD-IPMNs without worrisome features[18]. EUS-FNA with cyst fluid CEA determination may also be required for the differentiation between BD-IPMN and oligocystic serous cystic neoplasm[18,75].
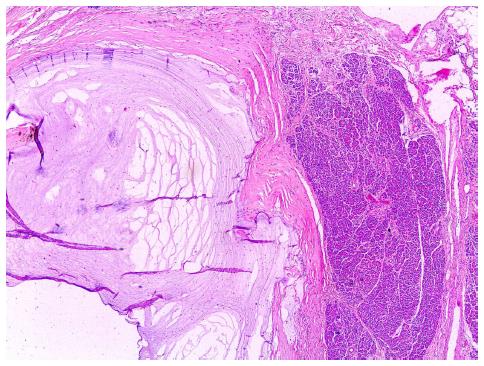
Focusing on the mucinous category, accurate distinction is not always possible between various mucinous cystic lesions. In a large series of resected mucin-producing neoplasms of the pancreas, 6% of mucinous cystic lesions were undetermined[22]. BD type of IPMN, especially when largely flat, can be confused with MCN if the topological branch ducts relationship is not clear. In addition, although MCN lacks a connection to the duct system, it rarely can fistulize into the ducts and very rarely exhibits intracystic papillary-like growth that may be confused with papillary structures of MD-IPMN[82] (Figure 10A). Ovarian-type stroma in MCN facilitates this differential diagnosis and accentuates the clinical distinction of MCN vs IPMN[7] (Figure 10B). MCN occurs in patients usually younger than BD type of IPMN (44.5 years vs 66 years in a large series[22]) and it is almost always a single lesion located in the pancreatic body/tail in women, whereas this type of IPMN occurs more commonly in the pancreatic head, can be single or multifocal, and occurs slightly more often in men[7,22]. Cystic mucin-producing pancreatic neoplasms without either IPMN histological features or ovarian-type stroma are better termed indeterminate mucin-producing neoplasms[7]. If such indeterminate cystic lesions exhibit simple mucinous epithelium without cytological atypia, they still are considered neoplasms by some authors[64]. Alternatively, they are termed non neoplastic (or non dysplastic) cystic mucinous lesions by others[83], although it is unclear whether they could represent the earliest manifestation of mucinous neoplasms. Retention cysts should also be considered. They happen because of pancreatic duct obstruction. They usually are unilocular and lined by normal or flattened ductal epithelium without atypia, but sometimes they are described with slight papillary or mucinous change[4]. Therefore, there are no specific limits for the distinction between retention cyst, non neoplastic mucinous cyst and some neoplastic mucinous cysts.
PanIN is the other main premalignant lesion of the pancreas besides IPMN. Lesions of PanIN can be flat, micropapillary or papillary, but unlike IPMN which is macroscopically visible, PanIN is defined as a microscopic entity[8]. Although PanIN lesions typically arise in the smaller ducts, it may involve large ducts. In addition, IPMN often extends from larger ducts to smaller pancreatic ducts[4]. The histological distinction between IPMN and PanIN is not always possible. The main issue concerns BD gastric type of IPMN because of its peripheral location and more similar cytohistological appearance and immunohistochemical profile (MUC2 negative and MUC5AC positive)[43,61]. It has generally been assumed that PanIN measures less than 0.5 cm and IPMN over 1 cm. It has been suggested to use a descriptive diagnosis, such as intraductal proliferative lesion of undetermined type, for an especially gray area of 0.5-1 cm featureless diameter[61].
Intraductal tubulopapillary neoplasm (ITPN) is a rare lesion characterized by a more solid intraductal growth without visible mucin secretions and with less cystic aspect than IPMN. Histologically, ITPN is characterized by a complex proliferation of tubules and variable extension of papillary architecture. Neoplastic cells show scant cytoplasmic mucin and uniform high grade dysplasia. Solid areas and necrotic foci are frequently seen. Associated invasive carcinoma is frequently scarce and observed in about 40% of cases[1,84]. ITPN is considered within the spectrum of IPMNs by some authors, although it is regarded as a separate entity by the current WHO system[16].
P- Reviewer: Fan Y, Memon MA S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Bosman FT, Carneiro F, Hruban RH, Theise ND (eds). WHO Classification of Tumors of the Digestive System. Lyon: IARC Press 1997; 279-337. [Cited in This Article: ] |
| 2. | Hamilton SR, Aaltonen LA (eds). WHO Classification of Tumors. Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC Press 2000; 219-251. [Cited in This Article: ] |
| 3. | Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 463] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 4. | Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 788] [Cited by in F6Publishing: 705] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 5. | Maguchi H, Tanno S, Mizuno N, Hanada K, Kobayashi G, Hatori T, Sadakari Y, Yamaguchi T, Tobita K, Doi R. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011;40:364-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Fernández-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139:708-713; 713.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1539] [Cited by in F6Publishing: 1413] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 8. | Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579-586. [PubMed] [Cited in This Article: ] |
| 9. | Ohashi K, Murakami Y, Maruyama M. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater [in japanese]. Prog Dig Endoscopy. 1982;20:348–351. [Cited in This Article: ] |
| 10. | Yanagisawa A, Ohashi K, Hori M, Takagi K, Kitagawa T, Sugano H, Kato Y. Ductectatic-type mucinous cystadenoma and cystadenocarcinoma of the human pancreas: a novel clinicopathological entity. Jpn J Cancer Res. 1993;84:474-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Rogers PN, Seywright MM, Murray WR. Diffuse villous adenoma of the pancreatic duct. Pancreas. 1987;2:727-730. [PubMed] [Cited in This Article: ] |
| 12. | Morohoshi T, Kanda M, Asanuma K, Klöppel G. Intraductal papillary neoplasms of the pancreas. A clinicopathologic study of six patients. Cancer. 1989;64:1329-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327-338. [PubMed] [Cited in This Article: ] |
| 14. | Adsay V, Jang KT, Roa JC, Dursun N, Ohike N, Bagci P, Basturk O, Bandyopadhyay S, Cheng JD, Sarmiento JM. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are ≥1.0 cm): clinicopathologic and immunohistochemical analysis of 123 cases. Am J Surg Pathol. 2012;36:1279-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Ohike N, Kim GE, Tajiri T, Krasinskas A, Basturk O, Coban I, Bandyopadhyay S, Morohoshi T, Goodman M, Kooby DA. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34:1731-1748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol. 2012;43:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Hubran RH, Pitman MB, Klimstra DS. Tumors of the Pancreas. Atlas of Tumor Pathology. 4th ed. Washington DC: Am Registry Pathol AFIP 2007; 75-110. [Cited in This Article: ] |
| 18. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1714] [Cited by in F6Publishing: 1575] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 19. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 643] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 20. | Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, Chari ST, Farnell MB. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639-646; discussion 646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 175] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 22. | Crippa S, Fernández-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Domínguez I, Muzikansky A, Thayer SP, Falconi M, Mino-Kenudson M. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 255] [Article Influence: 18.2] [Reference Citation Analysis (2)] |
| 23. | Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509-516. [PubMed] [Cited in This Article: ] |
| 24. | Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Nara S, Shimada K, Kosuge T, Kanai Y, Hiraoka N. Minimally invasive intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic study of 104 intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2008;32:243-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Salvia R, Malleo G, Marchegiani G, Pennacchio S, Paiella S, Paini M, Pea A, Butturini G, Pederzoli P, Bassi C. Pancreatic resections for cystic neoplasms: from the surgeon’s presumption to the pathologist’s reality. Surgery. 2012;152:S135-S142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernández-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010;10:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Shimizu Y, Yamaue H, Maguchi H, Yamao K, Hirono S, Osanai M, Hijioka S, Hosoda W, Nakamura Y, Shinohara T. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas. 2013;42:883-888. [PubMed] [Cited in This Article: ] |
| 29. | Lafemina J, Katabi N, Klimstra D, Correa-Gallego C, Gaujoux S, Kingham TP, Dematteo RP, Fong Y, D’Angelica MI, Jarnagin WR. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Ann Surg Oncol. 2013;20:440-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Salvia R, Crippa S, Partelli S, Armatura G, Malleo G, Paini M, Pea A, Bassi C. Differences between main-duct and branch-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:342-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678-685; discussion 685-687. [PubMed] [Cited in This Article: ] |
| 32. | Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72-79; quiz 309-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 33. | Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Fléjou JF. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372-1377. [PubMed] [Cited in This Article: ] |
| 34. | Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Obana T, Koshida S, Kanno Y, Yamashita Y, Kato Y. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45:1080-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Yamada Y, Mori H, Hijiya N, Matsumoto S, Takaji R, Ohta M, Kitano S, Moriyama M. Intraductal papillary mucinous neoplasms of the pancreas complicated with intraductal hemorrhage, perforation, and fistula formation: CT and MR imaging findings with pathologic correlation. Abdom Imaging. 2012;37:100-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Rosenberger LH, Stein LH, Witkiewicz AK, Kennedy EP, Yeo CJ. Intraductal papillary mucinous neoplasm (IPMN) with extra-pancreatic mucin: a case series and review of the literature. J Gastrointest Surg. 2012;16:762-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Imaoka H, Yamao K, Salem AA, Mizuno N, Takahashi K, Sawaki A, Isaka T, Okamoto Y, Yanagisawa A, Shimizu Y. Pseudomyxoma peritonei caused by acute pancreatitis in intraductal papillary mucinous carcinoma of the pancreas. Pancreas. 2006;32:223-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Zapiach M, Yadav D, Smyrk TC, Fletcher JG, Pearson RK, Clain JE, Farnell MB, Chari ST. Calcifying obstructive pancreatitis: a study of intraductal papillary mucinous neoplasm associated with pancreatic calcification. Clin Gastroenterol Hepatol. 2004;2:57-63. [PubMed] [Cited in This Article: ] |
| 39. | Kalaitzakis E, Braden B, Trivedi P, Sharifi Y, Chapman R. Intraductal papillary mucinous neoplasm in chronic calcifying pancreatitis: egg or hen? World J Gastroenterol. 2009;15:1273-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Furukawa T, Takahashi T, Kobari M, Matsuno S. The mucus-hypersecreting tumor of the pancreas. Development and extension visualized by three-dimensional computerized mapping. Cancer. 1992;70:1505-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 41. | D’Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 42. | Kang MJ, Lee KB, Jang JY, Han IW, Kim SW. Evaluation of clinical meaning of histological subtypes of intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2013;42:959-966. [PubMed] [Cited in This Article: ] |
| 43. | Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Ban S, Naitoh Y, Mino-Kenudson M, Sakurai T, Kuroda M, Koyama I, Lauwers GY, Shimizu M. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol. 2006;30:1561-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Ishida M, Egawa S, Aoki T, Sakata N, Mikami Y, Motoi F, Abe T, Fukuyama S, Sunamura M, Unno M. Characteristic clinicopathological features of the types of intraductal papillary-mucinous neoplasms of the pancreas. Pancreas. 2007;35:348-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Takasu N, Kimura W, Moriya T, Hirai I, Takeshita A, Kamio Y, Nomura T. Intraductal papillary-mucinous neoplasms of the gastric and intestinal types may have less malignant potential than the pancreatobiliary type. Pancreas. 2010;39:604-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Kim J, Jang KT, Mo Park S, Lim SW, Kim JH, Lee KH, Lee JK, Heo JS, Choi SH, Choi DW. Prognostic relevance of pathologic subtypes and minimal invasion in intraductal papillary mucinous neoplasms of the pancreas. Tumour Biol. 2011;32:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Basturk O, Khayyata S, Klimstra DS, Hruban RH, Zamboni G, Coban I, Adsay NV. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 2010;34:364-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, Nagata K, Tanaka S, Sakoda K, Aikou T. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Yonezawa S, Higashi M, Yamada N, Goto M. Precursor lesions of pancreatic cancer. Gut Liver. 2008;2:137-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 393] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 52. | Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1288] [Cited by in F6Publishing: 1330] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 53. | Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:108-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Yeh TS, Ho YP, Chiu CT, Chen TC, Jan YY, Chen MF. Aberrant expression of cdx2 homeobox gene in intraductal papillary-mucinous neoplasm of the pancreas but not in pancreatic ductal adenocarcinoma. Pancreas. 2005;30:233-238. [PubMed] [Cited in This Article: ] |
| 55. | Adsay NV, Adair CF, Heffess CS, Klimstra DS. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980-994. [PubMed] [Cited in This Article: ] |
| 56. | Liszka L, Pajak J, Zielińska-Pajak E, Krzych L, Gołka D, Mrowiec S, Lampe P. Intraductal oncocytic papillary neoplasms of the pancreas and bile ducts: a description of five new cases and review based on a systematic survey of the literature. J Hepatobiliary Pancreat Sci. 2010;17:246-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Lüttges J, Zamboni G, Longnecker D, Klöppel G. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942-948. [PubMed] [Cited in This Article: ] |
| 58. | Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, Fernández-Del Castillo C, Warshaw AL, Thayer SP. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology. 2010;138:1166-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 59. | Sauvanet A, Couvelard A, Belghiti J. Role of frozen section assessment for intraductal papillary and mucinous tumor of the pancreas. World J Gastrointest Surg. 2010;2:352-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Couvelard A, Sauvanet A, Kianmanesh R, Hammel P, Colnot N, Lévy P, Ruszniewski P, Bedossa P, Belghiti J. Frozen sectioning of the pancreatic cut surface during resection of intraductal papillary mucinous neoplasms of the pancreas is useful and reliable: a prospective evaluation. Ann Surg. 2005;242:774-778, discussion 778-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Longnecker DS, Adsay NV, Fernandez-del Castillo C, Hruban RH, Kasugai T, Klimstra DS, Klöppel G, Lüttges J, Memoli VA, Tosteson TD. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas. 2005;31:344-349. [PubMed] [Cited in This Article: ] |
| 62. | Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology. 2002;2:484-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 63. | Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 64. | Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 10] [Reference Citation Analysis (0)] |
| 65. | Seidel G, Zahurak M, Iacobuzio-Donahue C, Sohn TA, Adsay NV, Yeo CJ, Lillemoe KD, Cameron JL, Hruban RH, Wilentz RE. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol. 2002;26:56-63. [PubMed] [Cited in This Article: ] |
| 66. | Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, Brennan MF, Klimstra DS. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26-42. [PubMed] [Cited in This Article: ] |
| 67. | Maire F, Hammel P, Terris B, Paye F, Scoazec JY, Cellier C, Barthet M, O’Toole D, Rufat P, Partensky C. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 68. | Woo SM, Ryu JK, Lee SH, Yoo JW, Park JK, Kim YT, Yoon YB. Survival and prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas: comparison with pancreatic ductal adenocarcinoma. Pancreas. 2008;36:50-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, Jain A, Edil BH, Iacobuzio-Donahue CA, Schulick RD. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 70. | Nakagohri T, Asano T, Kenmochi T, Urashima T, Ochiai T. Long-term surgical outcome of noninvasive and minimally invasive intraductal papillary mucinous adenocarcinoma of the pancreas. World J Surg. 2002;26:1166-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Takahashi H, Nakamori S, Nakahira S, Tsujie M, Takahshi Y, Marubashi S, Miyamoto A, Takeda Y, Nagano H, Dono K. Surgical outcomes of noninvasive and minimally invasive intraductal papillary-mucinous neoplasms of the pancreas. Ann Surg Oncol. 2006;13:955-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Nakata K, Ohuchida K, Aishima S, Sadakari Y, Kayashima T, Miyasaka Y, Nagai E, Mizumoto K, Tanaka M, Tsuneyoshi M. Invasive carcinoma derived from intestinal-type intraductal papillary mucinous neoplasm is associated with minimal invasion, colloid carcinoma, and less invasive behavior, leading to a better prognosis. Pancreas. 2011;40:581-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Stelow EB, Pambuccian SE, Bauer TW, Moskaluk CA, Klimstra DS. Mucus rupture (extrusion) and duct expansion/expansive growth are not diagnostic of minimal invasion when seen with intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:320-321; author reply 321-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Kelly PJ, Shinagare S, Sainani N, Hong X, Ferrone C, Yilmaz O, Fernández-del Castillo C, Lauwers GY, Deshpande V. Cystic papillary pattern in pancreatic ductal adenocarcinoma: a heretofore undescribed morphologic pattern that mimics intraductal papillary mucinous carcinoma. Am J Surg Pathol. 2012;36:696-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | O’Toole D, Palazzo L, Hammel P, Ben Yaghlene L, Couvelard A, Felce-Dachez M, Fabre M, Dancour A, Aubert A, Sauvanet A. Macrocystic pancreatic cystadenoma: The role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc. 2004;59:823-829. [PubMed] [Cited in This Article: ] |
| 76. | Kawamoto S, Scudiere J, Hruban RH, Wolfgang CL, Cameron JL, Fishman EK. Solid-pseudopapillary neoplasm of the pancreas: spectrum of findings on multidetector CT. Clin Imaging. 2011;35:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Basturk O, Zamboni G, Klimstra DS, Capelli P, Andea A, Kamel NS, Adsay NV. Intraductal and papillary variants of acinar cell carcinomas: a new addition to the challenging differential diagnosis of intraductal neoplasms. Am J Surg Pathol. 2007;31:363-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Yoon WJ, Daglilar ES, Pitman MB, Brugge WR. Cystic pancreatic neuroendocrine tumors: endoscopic ultrasound and fine-needle aspiration characteristics. Endoscopy. 2013;45:189-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Khashab MA, Kim K, Lennon AM, Shin EJ, Tignor AS, Amateau SK, Singh VK, Wolfgang CL, Hruban RH, Canto MI. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas. 2013;42:717-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 80. | Pitman MB, Genevay M, Yaeger K, Chebib I, Turner BG, Mino-Kenudson M, Brugge WR. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than “positive” cytology. Cancer Cytopathol. 2010;118:434-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 81. | Genevay M, Mino-Kenudson M, Yaeger K, Konstantinidis IT, Ferrone CR, Thayer S, Castillo CF, Sahani D, Bounds B, Forcione D. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg. 2011;254:977-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 82. | Masia R, Mino-Kenudson M, Warshaw AL, Pitman MB, Misdraji J. Pancreatic mucinous cystic neoplasm of the main pancreatic duct. Arch Pathol Lab Med. 2011;135:264-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 83. | Nadig SN, Pedrosa I, Goldsmith JD, Callery MP, Vollmer CM. Clinical implications of mucinous nonneoplastic cysts of the pancreas. Pancreas. 2012;41:441-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Yamaguchi H, Shimizu M, Ban S, Koyama I, Hatori T, Fujita I, Yamamoto M, Kawamura S, Kobayashi M, Ishida K. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |