Published online Jun 15, 2010. doi: 10.4251/wjgo.v2.i6.251
Revised: January 16, 2010
Accepted: January 23, 2010
Published online: June 15, 2010
Goblet cell carcinoid is an enigmatic and rare tumor involving the appendix almost exclusively. Since its identification in 1969, understanding of this disease has evolved greatly, but issues regarding its histogenesis, nomenclature and management are still conjectural. The published English language literature from 1966 to 2009 was retrieved via PubMed and reviewed. Various other names have been used for this entity such as adenocarcinoid, mucinous carcinoid, crypt cell carcinoma, and mucin-producing neuroendocrine tumor, although none have been found to be completely satisfactory or universally accepted. The tumor is thought to arise from pluripotent intestinal epithelial crypt-base stem cells by dual neuroendocrine and mucinous differentiation. GCCs present in the fifth to sixth decade and show no definite sex predominance. The most common clinical presentation is acute appendicitis, followed by abdominal pain and a mass. Fifty percent of the female patients present with ovarian metastases. The histologic hallmark of this entity is the presence of clusters of goblet cells in the lamina propria or submucosa stain for various neuroendocrine markers, though the intensity is often patchy. Atypia is usually minimal, but carcinomatous growth patterns may be seen. These may be of signet ring cell type or poorly differentiated adenocarcinoma. Recently molecular studies have shown these tumors to lack the signatures of adenocarcinoma but they have some changes similar to that of ileal carcinoids (allelic loss of chromosome 11q, 16q and 18q). The natural history of GCC is intermediate between carcinoids and adenocarcinomas of the appendix. The 5-year overall survival is 76%. The most important prognostic factor is the stage of disease. Appendectomy and right hemicolectomy are the main modalities of treatment, followed by adjuvant chemotherapy in select cases. There is some debate about the surgical approach for these tumors, and a summary of published series and recommendations are provided.
- Citation: Roy P, Chetty R. Goblet cell carcinoid tumors of the appendix: An overview. World J Gastrointest Oncol 2010; 2(6): 251-258
- URL: https://www.wjgnet.com/1948-5204/full/v2/i6/251.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v2.i6.251
Goblet cell carcinoid (GCC) tumors are a unique and distinctive tumor type that occurs almost exclusively in the appendix with rare cases encountered outside this location. With its distinctive histologic appearance and variable biologic behavior it has been the source of debate amongst pathologists and surgeons alike. The purpose of this review is to highlight the origins and general features of the tumor, discuss the nomenclatural difficulties associated with the continued use of the suffix “carcinoid”, provide a brief overview of the pathology including immunohistochemical and molecular aspects and, finally, to dwell on management and prognostic issues.
This narrative review is based on a literature search of Index Medicus using the PubMed search engine. A comprehensive search was performed for all articles between 1966 to the present using the search terms “GCC”, “adenocarcinoid”, “mucinous carcinoid”, “crypt cell carcinoma” and “appendiceal neoplasms”. Both free text search and MeSH based searches were performed. Additional searches were performed for literature on differential diagnoses including carcinoid tumors and colonic-type adenocarcinomas using appropriate search terms.
The review extracts information from the results of this search of published peer-reviewed literature to focus on the evolution of this unique histopathological entity; its pathological aspects, nomenclature and classification; the epidemiology of this disease; its management dilemmas, outcomes and prognostic factors.
The first description of a tumor of the appendix distinct from both adenocarcinomas and carcinoids was made by Gagne et al[1] in the late 1960s. Subsequently, several reports described this entity although the names ascribed to it have differed.
In 1974, Subbuswamy et al[2] first coined the term “GCC” in reporting a series of 12 cases of this new entity, as the principal cell type morphologically resembled goblet cells of the gastrointestinal tract, while having a significant population of argentaffin cells. In the same year Klein[3] reported 3 cases of a similar tumor which they termed mucinous carcinoid tumor. Warkel et al[4]reported the first large series of 39 cases and preferred the term “adenocarcinoid”. This was also the first study that documented this entity as a distinct prognostic group intermediate between carcinoids and the classical adenocarcinoma. These initial reports suggested that this tumor was a subtype of carcinoid tumor on the basis of the basil-glandular/mural location, its well differentiated nature, and the absence of dysplasia in the surrounding epithelium[2]. Further studies suggested various other names such as “microglandular carcinoma”, “intermediate cell carcinoid”, “amphicrine neoplasia” and “composite tumor”, and raised questions about the histogenesis of this entity. However to date no consensus has been reached, and whether GCC is a variant of neuroendocrine tumor or a subtype of adenocarcinoma with neuroendocrine differentiation is still a subject of debate.
More detailed immunohistochemical and molecular analyses raised have now greatly improved our understanding of this entity and led to its incorporation into standard classification systems.
The histogenetic evolution of this tumor has been subject to hypothesis in the last three decades, pioneered by Warner et al[5] in 1979. Isaacson[6] demonstrated IgA, a secretory component and lysozyme in these tumors , similar to intestinal crypt cells, and proposed the name “crypt cell carcinoma”. It is believed that GCC represents an amphicrine tumor, which originates from a single undifferentiated pluripotent intestinal epithelial crypt base progenitor stem cell with divergent neuroendocrine and mucinous glandular differentiation. This cell type is not usually present in appendiceal mucosa and is thought to be generated in a similar method to Paneth cell metaplasia[6]. This concept was reiterated by Ratzenhofer et al[7] and more recently by Goddard et al[8]. Rare case reports of GCC combined with mucinous cystadenoma suggest an adenoma-carcinoma sequence[9]. Classical carcinoids in contrast, have been shown to have a mono-lineage differentiation from subepithelial neuroendocrine cells in the lamina propria. If GCC is a true subtype of carcinoid tumor, its coexistence with an appendiceal mucinous neoplasm supports the theory that GCC is derived from a pluripotent intestinal stem cell with divergent dual neuroendocrine and mucinous differentiation (unitary intestinal stem cell theory)[9].
The use of the appellation “carcinoid” for this tumor is disliked by many due to the presumed benign connotation. Moreover, most GCCs have been shown to stain inconsistently with neuroendocrine markers and contain very few endocrine cells (APUD cells). Recent studies based on immunohistochemical and molecular findings have shown CEA, CDX2, CK7 and CK20 expression in these tumors, similar to that of colonic adenocarcinomas[10-12]. Unfortunately, arguments against calling it a type of adenocarcinoma are as many as those in favour. Unlike adenocarcinomas, K-ras, and β-catenin expression is absent in these tumors[13,14]. These tumors also show allelic loss of chromosomes 11q, 16q and 18q, similar to ileal carcinoids.
In view of the molecular signatures of this entity, and keeping in mind the amphicrine histogenesis, we feel there is enough evidence for a neuroendocrine link in these tumors. We thus propose the name “mucin-producing neuroendocrine tumor (or carcinoma) of the appendix”.
The World Health Organization accepted the term ‘GCC’ and “mucinous carcinoid”, but identified it as distinct from both carcinoids and adenocarcinoma in the classification of epithelial appendiceal tumors (International Classification for Disease, ICD-O 8243/3). They segregated mixed carcinoid-adenocarcinoma, and tubular carcinoids as separate entities[15]. Tubular carcinoids are small gland-forming tumors, with similar pattern of infiltration to carcinoids, and may have focal mucin in them. These were originally included under the broad category of adenocarcinoids, but are now considered a sub-type of typical carcinoids[4,16].
Recently, in a publication of a large series of 61 cases, Tang et al[17] suggested a broader spectrum of tumours to be included under this eponym, as typical GCCs have the potential to transform into signet ring or poorly differentiated carcinomas. They suggested classification into 3 groups-typical GCC (Group A), and adenocarcinoma ex-GCC, which was further divided into signet ring cell type (Group B), and poorly differentiated adenocarcinoma type (Group C).
GCC of the appendix is a rare tumor constituting 5% of all primary appendiceal neoplasms[18]. The age-adjusted annual incidence of appendiceal malignancies is 0.12 cases per million[19,20].
The age of presentation ranges from 18 to 89 years with the majority of patients presenting in their fifth or sixth decades[14,19,21]. This contrasts with the average age of presentation for malignant carcinoids (38 years) and adenocarcinoma of the appendix (62 years)[19]. The reported male to female ratio has varied in literature between 1.4:1 to 1:2.2[9,17,19]. It is more commonly seen in Caucasians[19].
GCCs are unique to the appendix. The most common clinical presentation is acute appendicitis[4,20,22]. However, Tang et al[17] reported abdominal pain and lower abdominal palpable mass in 50% patients, while acute appendicitis was the presenting feature in 44%. Half the female patients present with metastases to the ovary[22]. Tang et al[17] noted that 83% of female stage IV patients presented with ovarian masses and had a presumed preoperative diagnosis of a primary ovarian tumor.
Other presenting symptoms are bowel obstruction, intussusception, gastrointestinal bleeding, chronic intermittent lower abdominal pain and non-specific rare presentations such as mesenteric adenitis, intestinal obstruction and iron deficiency anemia due to cecal ulceration[9,22]. Incidental finding of GCC is reported in 3%[17].
Presentation with stage III/IV disease is reported at 51% to 97%[17,19,22]. Tang et al[17] reported an incidence of 63% for patients presenting as stage IV with metastasis, while others reported it at as low as 14%. This discrepancy between authors might be explained by the fact that different authors included different morphologic spectrums of this disease in the diagnosis, and many only included the more indolent tumors, excluding the aggressive variant as an adenocarcinoma.
The most common route of metastases is trans-coelomic/peritoneal invasion, and the most common sites involved are ovaries, and the peritoneal surfaces of the pelvis and abdominal cavity. Metastasis to the ribs, vertebra, and lymph nodes have also been reported[23]. Lymph node metastasis was detected in 17% to 38% of patients and involvement of mesenteric nodes was limited to locally advanced tumors[17,19]. Rare sites of metastasis, like the prostate, have also been reported[24].
Pham et al[22] and Varisco et al[23] have demonstrated similarities between this disease with ovarian neoplasms and appendiceal mucinous cystadenocarcinoma in being indolent diseases with similar modes of dissemination and survival outcomes.
None of these patients present with carcinoid syndrome, and urinary 5HIAA levels in these patients are usually within normal limits[21]. Ten percent of patients have been reported to have other malignancies along with GCC[22].
Gross finding of a discrete mass is very rare for these tumors. They usually appear as ill-defined firm nodular thickening of the appendix. Majority of the tumors are > 2 cm in size, the average being 2.4 cm[17,18]. However, tumor size is difficult to measure grossly due to the diffuse pattern of infiltration. Most commonly the lesion is noted in the tip of the appendix, followed by the base. Circumferential involvement of appendiceal wall with longitudinal extension is the most common growth pattern. For proper evaluation of this lesion, it is recommended that the entire appendix should be submitted[17].
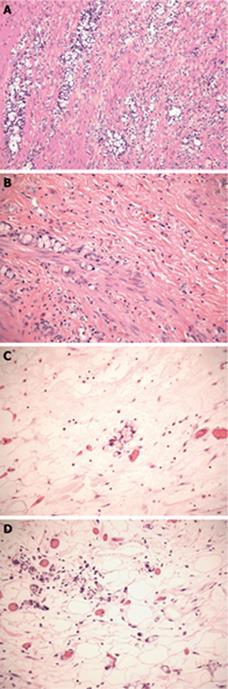
When Subbuswamy et al[2] first described this entity, he identified a tumor in which mucin filled cells with crescentic nuclei were arranged in small clumps or rosettes, with no definite lumen (Figure 1A). He noted the striking resemblance to goblet cells or signet ring cells, with the clumps resembling Brunner’s glands. The bulk of the tumor is in the lamina propria or submucosal layer, surrounding the basil-glandular crypts. Most clusters are formed of 4 to 15 cell groups, and are widely separated by stroma (Figure 1B). In addition, there is another distinct component of small to intermediate cells, with eosinophilic finely vacuolar or granular cytoplasm, vesicular nuclei, prominent nucleoli, and mild cytologic atypia, reminiscent of typical carcinoids. Numerous Paneth cells may also be seen. Occasionally goblet cell nests are found in large pools of mucin in the muscularis propria (Figure 1C). Atypia is usually minimal, but the tumor can have areas with carcinomatous, high-grade appearance. Burke et al[25] defined dominant carcinomatous growth patterns as fused or cribriform glands, single file structures, diffusely infiltrating signet ring cells, sheets of tumor cells, compressed goblet cell nests with small glands, signet ring cells with little or no intervening stroma, and extracellular mucin pools with fused glandular epithelium lacking lumina. Mitotic figures are rare, and reach up to 4/10 high power fields (hpf) in lesions that are high-grade and metastatic[4]. Vascular and perineural invasion, and infiltration of periappendiceal fat are common findings (Figure 1D). The surrounding epithelium is either well-preserved or shows fibrous obliteration of the lumen without evidence of adenomatous change. Associated acute appendicitis was noted in some cases[4].
Tang et al[17] have advocated classifying these tumors in one of three morphologic patterns to help assess prognosis. Typical GCC or group A comprises well defined goblet cells arranged in clusters with cohesive linear pattern, minimal cytologic atypia or architectural distortion of appendiceal wall and no desmoplasia. Cases showing degenerative changes with extracellular mucin are included in this group. Adenocarcinoma ex-GCC, signet ring cell type or group B cases have discohesive cells with signet ring cell features and significant cytologic atypia. There is single cell infiltration pattern with prominent desmoplasia and associated destruction of appendiceal wall. Group C or adenocarcinoma ex GCC, poorly differentiated carcinoma type, has a component (> 1/hpf or 1 mm2) not otherwise distinguishable from a poorly differentiated adenocarcinoma, which may appear as gland-forming confluent sheets of signet ring cells, or undifferentiated carcinoma, along with at least focal evidence of goblet cell morphology. Wang et al[26] have questioned the diagnostic reproducibility of this system due to the difficulty involved in distinguishing goblet cells from signet ring cells. They also disapproved of the nomenclature, as the term signet ring cell carcinoma generally connotes a poorly differentiated carcinoma and is less than ideal in differentiating groups B and C.
Most cells are argyrophil-positive (Sevier-Munger stain), but rarely argentaffin-positive (with Fontana-Masson stain). All vacuolated cells are positive for mucicarmine, PAS, PAS with diastase and Alcian blue[27].
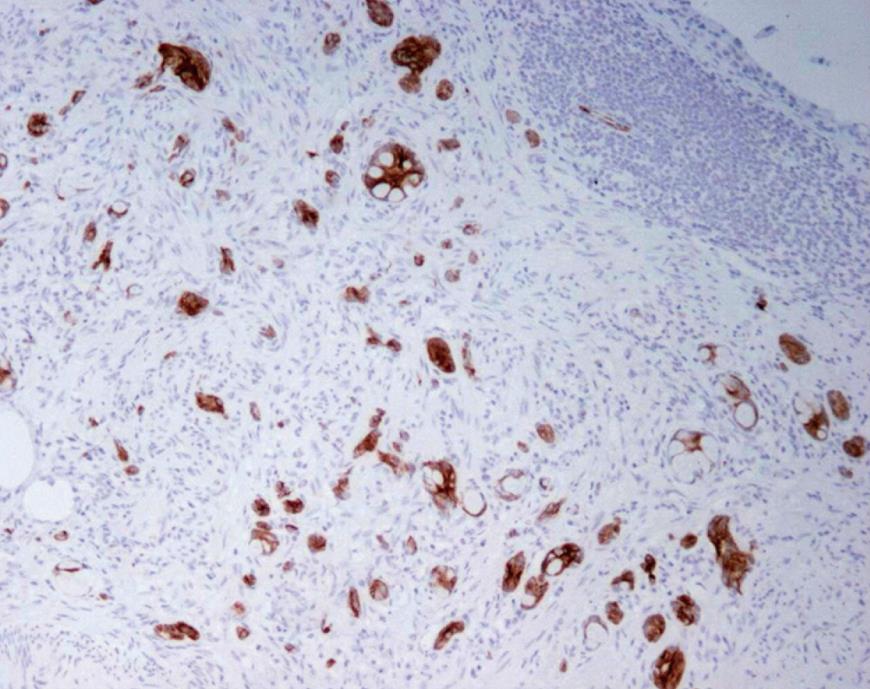
These tumors generally show strong CEA, CDX-2 CAM 5.2 and CK positivity, and are inconsistently positive for neuroendocrine markers[10]. CK20 positivity has been noted in 100% (Figure 2) and CK7 in 70.5%, while carcinoids are negative for CK7, with reports of up to 16% positivity for CK20[11]. CK19 staining is positive in GCC, and does not correlate with prognosis, unlike that in gastrointestinal and pulmonary neuroendocrine tumors[28]. A significant proportion of classical (non-goblet cell) carcinoids also stain for this marker. Both these tumors show similar positivity for CD99 in 70% cases, with no relation with prognosis[28]. Normal large gut mucosa express MUC2 only, similar to Group A and B GCC. In adenocarcinoma, MUC2 is lost and MUC1 over expressed, as is for Group C GCC[17].
Expression of neuroendocrine markers is variable and present in up to 50% of cells. One or more neuroendocrine markers are always positive in these tumors. These include synaptophysin, chromogranin A, somatostatin, serotonin, neuron specific enolase, PGP9.5, and pancreatic polypeptide. However, the pattern of positivity is focal and patchy, unlike that in carcinoid tumors, where it is strong and diffuse[17]. Bombesin, endorphin, gastrin and secretin were found to be negative[29].
S100, Leu7 (CD57) and NCAM (CD56) staining around tumor cell nests is usually not identified in these tumors (unlike carcinoids)[8]. Sometimes, entrapped nerves may give an erroneous impression of positive stain[10]. The unpredictable behavior of this tumor may be explained by the increased proliferation despite lack of visible mitoses. Proliferating cell nuclear antigen (PCNA) positivity has been reported at 60%-90%[30]. Ki-67 (MIB-1) immunolabeling varied from 0% to 80% in different tumors, with index
> 2% noted in 41.1% of GCC[11,17]. Alsaad et al[11] did not find any correlation of Ki-67 with prognosis, though Li et al[31] reported a worse prognosis for those with Ki-67 labeling index of > 3%.
p53 and p16 are reported to be negative in most cases, suggesting a p53 independent pathway in this tumor. Dysregulation of the cell cycle pathway is likely to be involved, as suggested by over expression of cyclin D1 (30%-70%) and p21 (40%-60%)[13,14,30].
β-catenin staining in GCC is similar to normal appendiceal mucosa and typical carcinoid, with strong membranous staining but without nuclear or cytoplasmic staining[31]. In adenocarcinoma, β-catenin stain is usually nuclear, although it may also be diffuse cytoplasmic, with reduced or absent membranous staining. E-cadherin staining is also strong membranous in GCC. This is similar to carcinoids and unlike adenocarcinoma where E-cadherin is negative[31].
Rb protein expression is preserved in GCC[17]. DPC4 (Smad4) protein shows normal expression. HD5 and Math1 show scattered nuclear positivity in GCC similar to adenocarcinoma, which is negative in carcinoid[10].
Molecular studies have failed to conclusively settle the issue of histogenesis of these tumors. Different authors have noted conflicting results, although most indicate similarity between ileal carcinoids and GCC. Stancu et al[14] found allelic loss of chromosome 11q, 16q and 18q in 11%, 11% and 39% of GCCs, respectively. This is similar to ileal carcinoids (27%, 37% and 56%), but not to appendiceal carcinoids. They did not find mutations in K-ras, DPC-4 (Smad4) or β-catenin, which are usually present in colonic adenocarcinoma, but absent in carcinoid tumors. Ramnani et al[13] also similarly reported the lack of mutation of K-ras in these tumors, highlighting that the pathogenesis in these tumors involves a pathway other than ras oncogene. They however, reported p53 mutations in 25% of GCCs and 44% of classical carcinoids, suggesting this as a possible mechanism in some GCCs. The mutation did not correlate with positive immunohistochemical staining with p53. This is not surprising as it is well known that p53 mutation does not necessarily lead to protein overexpression, while p53 protein overexpression can take place in the absence of a genetic mutation.
Modlin et al[32] showed elevated expression of CgA, NAPIL1, MAGE-D2 and MTA1, along with decreased expression of NALP1 in GCC, compared to normal mucosa, and similar to malignant or small intestinal carcinoid. Further molecular studies are required to improve our understanding of this entity.
Electron microscopy shows small to large mucin droplets along with round/ovoid/elongated electron-dense neurosecretory granules in the neoplastic cells[30,33,34]. The granules contain chromogranin reaction products, and originating from ovoid or discoid EC2 type and D type, and are sometimes difficult to find[24,29].
Table 1 highlights the clinical and pathological differences between GCC, typical carcinoid tumor and adenocarcinoma of the appendix.
| Goblet cell carcinoid | Carcinoid | Adenocarcinoma | |
| Clinical features | |||
| Age | 5th-6th decade | 4th decade | 7th decade |
| Carcinoid syndrome | No | Yes | No |
| Primary symptoms | Acute appendicitis | Acute appendicitis | Mass |
| Gross appearance | > 2 cm, ill defined thickening | < 2 cm | > 2 cm, well defined mass |
| Microscopic appearance | |||
| Morphology | Clusters of goblet or signet-ring cells separated by fibrosis/pools of mucin | Nests of small cells | Well-formed glands to sheets of poorly differentiated signet-ring cells |
| Atypia | Minimal | Minimal | Marked |
| Mitosis | Rare | Rare | Increased |
| Vascular and perineural invasion | Present | Absent | Present |
| Infiltrative margins | Present | Absent | Present |
| Special stains | |||
| Argyrophil | Positive | Positive | Usually negative |
| Argentaffin | Negative | Positive | Negative |
| Mucicarmine/PAS/PASD | Positive in goblet cells | Negative | Positive |
| IHC | |||
| CEA | + | - | + |
| CDX2 | + | - | + |
| CAM5.2 | + | - | + |
| CK20 | ± | - | + |
| CK7 | ± | - | - |
| CK19 | + | - | - |
| Neuroendocrine markers | ± | ± | - |
| Math1 and HD5 | + | - | + |
| p53, p16 | - | - | + |
| Molecular pathology | |||
| DPC4, Kras, β-catenin mutation and p53 over expression | - | - | + |
Surgical resection is the primary mode of treatment for GCC. Overall, the natural history of this disease is intermediate in aggressiveness between classical adenocarcinomas and carcinoids. Reported 5-year survival rates have ranged between 58% and 83%[17]. Due to its rarity, however, there is a lack of robust evidence or high level consensus regarding the optimal extent of resection of different stages of this disease.
However, because of its natural history and malignant nature, treatment recommendations are in general similar to adenocarcinomas rather than classical carcinoids. Stage I tumors may be treated with appendectomy alone. In higher stages a right hemicolectomy (RH) is still the most commonly recommended surgical option, in spite of various controversies. The justification for RH is to do adequate nodal sampling, as GCC has shown a moderately high propensity for nodal metastasis[17]. Varisco et al[23] and Byrn et al[35] showed no residual tumor in the follow-up resections in cases with invasive GCC, highlighting the lack of benefit from extensive surgery in infiltrative tumors provided there was no nodal involvement. Pham et al[22] in their analysis of 58 patients showed that the 5-year survival rates were not significantly different between those treated with appendectomy and those who underwent right hemicolectomy. The SEER publication from 2004 showed that only 42% patients receive RH for GCC[19].
In loco-regionally advanced lesions, an extensive resection including oophorectomy or TAH-BSO may result in a reduction of peritoneal failure. Prophylactic removal of ovaries has also been suggested, especially for postmenopausal females wherever possible, due to the high incidence of ovarian metastases[17,22,35,36]. As a corollary, an appendectomy is recommended in patients presenting with Krukenberg’s tumors with unknown primary[37]. GCC has a propensity for peritoneal seeding, and those who die from this disease are thought to already have microscopic peritoneal disease at presentation. For extensive peritoneal spread cytoreductive surgery has been recommended on the lines of ovarian epithelial tumors.
A summary of recommendations based on reported evidence found in English literature has been compiled in Table 2 and reflects some of the inconsistencies prevalent in current practice.
| Subbuswamy et al[2] | APX |
| RH in case of cecal involvement | |
| Klein[3] | APX |
| Haqqani et al[40] | RH if base of appendix or caecum is involved |
| Warkel et al[4] | RH in case of spread beyond appendix, atypia, and mitotic count ≥ 2/10 hpf |
| Chen et al[41] | APX alone unless there is cecal involvement |
| Olsson et al[42] | RH in case of spread beyond appendix, atypia, and mitotic count ≥ 2/10 hpf |
| Edmonds et al[33] | RH in all cases |
| Bak et al[39] | RH in case of spread beyond appendix, atypia, and mitotic count ≥ 2/10 hpf |
| Park et al[43] | RH in all cases |
| Rutledge et al[44] | RH in all cases |
| Butler et al[36] | RH for cecal involvement, BSO in females |
| Ramnani et al[13] | < 2 cm in size, without serosal & lymphatic involvement-APX |
| More advanced tumor-RH | |
| Kanthan et al[30] | RH |
| Li et al[31] | RH for N1, M1 or Mib1 > 3% |
| Varisco et al[23] | RH in case of spread beyond appendix, atypia, and mitotic count ≥ 2/10 hpf |
| Byrn et al[35] | No value of RH in non-metastatic cases |
| Pham et al[22] | RH with attendant mesenteric nodal resection for (1) T3/T4 disease or nodal involvement; (2) direct cecal involvement; and (3) clinically positive mesenteric nodes |
| Bilateral oophorectomy for post menopausal women | |
| O’Donnell et al[20] | RH irrespective of stage |
| Tang et al[17] | Group A T1, 2-no recommendations |
| T3 or 4, group B/C, perforation, positive margin-RH with oophorectomy if possible. CT in stage III/IV | |
| Stage IV/group C- debulking/oophorectomy/systemic/intraperitoneal CT |
The choice of optimal adjuvant treatment after surgery also suffers from a lack of specific evidence. Chemotherapy with 5FU and leucovorin demonstrated a non-significant survival advantage over surgery alone in one study[22]. It is likely that most institutions approach this lesion with standard chemotherapy options for adenocarcinomas e.g. FOLFOX/FOLFIRI. Other chemotherapy options are streptozotocin with 5FU, cisplatin with etoposide, and interferon[38]. Intraperitoneal chemotherapy is considered in selected individuals for aggressive cytoreduction.
Long-term followup is advocated in view of the poor prognosis and recurrence. In-labeled octreotide scintigraphy is the most sensitive imaging modality for metastasis. FDG-PET is useful for high grade GCC. Plasma chromogranin A and urinary 5HIAA have also been used as biomarkers[38].
The prognosis of GCC is intermediate between carcinoids and appendiceal adenocarcinomas. According to the SEER data compiled between 1973 and 2001, the 5-year overall survival for GCC was 76%. The corresponding rates for localized, regional and distant disease were 86%, 74% and 18% respectively[18]. The baseline hazard ratio in comparison to colonic-type adenocarcinoma, stratified by age group and tumor extension is reported as 0.78 [95% confidence intervals (CI): 0.56 to 1.07]. In comparison the hazard ratio for malignant carcinoids is 0.56 (95% CI: 0.36 to 0.88)[19].
Stage is the most important prognostic factor. Pham reported 5-year disease specific survivals of 100%, 76%, 22% and 14% for stage I to IV according to the AJCC stage groups[22].
Tumor grade is also an important determinant of survival. Tang et al[17] reported mean survivals of 119, 43 and 31 mo for tumors in groups A, B and C respectively. The reported 5-year overall survival for their entire cohort was 77%.
Peritoneal carcinomatosis was the most common cause of death. The relationship of atypical histological features with prognosis is a matter of debate[25,39]. Histologic parameters predictive of aggressive behavior are high mitotic count (> 2/10 hpf), high Ki-67 index (> 3%), serosal or meso-appendiceal extension, angioinvasion, nodal involvement, increased number of Paneth cells, increased mucin secretion and production of pancreatic polypeptide[25,31,38]. However, others have shown that histological features do not correlate with prognosis[21]. Unlike carcinoids, tumor size is not predictive of outcome in GCC. Perineural and lymphatic invasion are common but do not correlate with prognosis[4].
Peer reviewer: Fahd Al-Mulla, PhD, Associate Professor, Department of Molecular Pathology, Kuwait University, Faculty of Medicine, Safat 13110, Kuwait
S- Editor Li LF L- Editor Hughes D E- Editor Yang C
| 1. | Gagné F, Fortin P, Dufour V, Delage C. [Tumors of the appendix associating histologic features of carcinoid and adenocarcinoma]. Ann Anat Pathol (Paris). 1969;14:393-406. [Cited in This Article: ] |
| 2. | Subbuswamy SG, Gibbs NM, Ross CF, Morson BC. Goblet cell carcinoid of the appendix. Cancer. 1974;34:338-344. [Cited in This Article: ] |
| 3. | Klein HZ. Mucinous carcinoid tumor of the vermiform appendix. Cancer. 1974;33:770-777. [Cited in This Article: ] |
| 4. | Warkel RL, Cooper PH, Helwig EB. Adenocarcinoid, a mucin-producing carcinoid tumor of the appendix: a study of 39 cases. Cancer. 1978;42:2781-2793. [Cited in This Article: ] |
| 5. | Warner TF, Seo IS. Goblet cell carcinoid of appendix: ultrastructural features and histogenetic aspects. Cancer. 1979;44:1700-1706. [Cited in This Article: ] |
| 6. | Isaacson P. Crypt cell carcinoma of the appendix (so-called adenocarcinoid tumor). Am J Surg Pathol. 1981;5:213-224. [Cited in This Article: ] |
| 7. | Ratzenhofer M, Auböck L. The amphicrine (endo-exocrine) cells in the human gut, with a short reference to amphicrine neoplasias. Acta Morphol Acad Sci Hung. 1980;28:37-58. [Cited in This Article: ] |
| 8. | Goddard MJ, Lonsdale RN. The histogenesis of appendiceal carcinoid tumours. Histopathology. 1992;20:345-349. [Cited in This Article: ] |
| 9. | Alsaad KO, Serra S, Chetty R. Combined goblet cell carcinoid and mucinous cystadenoma of the vermiform appendix. World J Gastroenterol. 2009;15:3431-3433. [Cited in This Article: ] |
| 10. | van Eeden S, Offerhaus GJ, Hart AA, Boerrigter L, Nederlof PM, Porter E, van Velthuysen ML. Goblet cell carcinoid of the appendix: a specific type of carcinoma. Histopathology. 2007;51:763-773. [Cited in This Article: ] |
| 11. | Alsaad KO, Serra S, Schmitt A, Perren A, Chetty R. Cytokeratins 7 and 20 immunoexpression profile in goblet cell and classical carcinoids of appendix. Endocr Pathol. 2007;18:16-22. [Cited in This Article: ] |
| 12. | Kende AI, Carr NJ, Sobin LH. Expression of cytokeratins 7 and 20 in carcinomas of the gastrointestinal tract. Histopathology. 2003;42:137-140. [Cited in This Article: ] |
| 13. | Ramnani DM, Wistuba II, Behrens C, Gazdar AF, Sobin LH, Albores-Saavedra J. K-ras and p53 mutations in the pathogenesis of classical and goblet cell carcinoids of the appendix. Cancer. 1999;86:14-21. [Cited in This Article: ] |
| 14. | Stancu M, Wu TT, Wallace C, Houlihan PS, Hamilton SR, Rashid A. Genetic alterations in goblet cell carcinoids of the vermiform appendix and comparison with gastrointestinal carcinoid tumors. Mod Pathol. 2003;16:1189-1198. [Cited in This Article: ] |
| 15. | Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumours of the Digestive System. World Health Organization Classification of Tumours. Lyon: IARC Press 2000; . [Cited in This Article: ] |
| 16. | Misdraji J. Neuroendocrine tumours of the appendix. Curr Diagn Pathol. 2005;11: 180-193. [Cited in This Article: ] |
| 17. | Tang LH, Shia J, Soslow RA, Dhall D, Wong WD, O'Reilly E, Qin J, Paty P, Weiser MR, Guillem J. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol. 2008;32:1429-1443. [Cited in This Article: ] |
| 18. | McGory ML, Maggard MA, Kang H, O'Connell JB, Ko CY. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48:2264-2271. [Cited in This Article: ] |
| 19. | McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94:3307-3312. [Cited in This Article: ] |
| 20. | O'Donnell ME, Carson J, Garstin WI. Surgical treatment of malignant carcinoid tumours of the appendix. Int J Clin Pract. 2007;61:431-437. [Cited in This Article: ] |
| 21. | Anderson NH, Somerville JE, Johnston CF, Hayes DM, Buchanan KD, Sloan JM. Appendiceal goblet cell carcinoids: a clinicopathological and immunohistochemical study. Histopathology. 1991;18:61-65. [Cited in This Article: ] |
| 22. | Pham TH, Wolff B, Abraham SC, Drelichman E. Surgical and chemotherapy treatment outcomes of goblet cell carcinoid: a tertiary cancer center experience. Ann Surg Oncol. 2006;13:370-376. [Cited in This Article: ] |
| 23. | Varisco B, McAlvin B, Dias J, Franga D. Adenocarcinoid of the appendix: is right hemicolectomy necessary? A meta-analysis of retrospective chart reviews. Am Surg. 2004;70:593-599. [Cited in This Article: ] |
| 24. | McGregor DH, Cherian R, Weston AP, Lawson L, McAnaw MP. Adenocarcinoid of ileum and appendix, incidentally discovered during exploratory laparotomy for gastric MALT lymphoma, with subsequent diffuse prostatic metastases: report of a case with light, immunohistochemical, and electron microscopic studies. Dig Dis Sci. 1999;44:87-95. [Cited in This Article: ] |
| 25. | Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB. Goblet cell carcinoids and related tumors of the vermiform appendix. Am J Clin Pathol. 1990;94:27-35. [Cited in This Article: ] |
| 26. | Wang HL, Dhall D. Goblet or signet ring cells: that is the question. Adv Anat Pathol. 2009;16:247-254. [Cited in This Article: ] |
| 27. | Pahlavan PS, Kanthan R. Goblet cell carcinoid of the appendix. World J Surg Oncol. 2005;3:36. [Cited in This Article: ] |
| 28. | Alsaad KO, Serra S, Perren A, Hsieh E, Chetty R. CK19 and CD99 immunoexpression profile in goblet cell (mucin-producing neuroendocrine tumors) and classical carcinoids of the vermiform appendix. Int J Surg Pathol. 2007;15:252-257. [Cited in This Article: ] |
| 29. | Gulubova MV, Yovchev Y, Vlaykova T, Hadjipetkov P, Prangova DK, Popharitov A. Application of light microscopical and ultrastructural immunohistochemistry in the study of goblet cell carcinoid in the appendix. World J Surg Oncol. 2008;6:15. [Cited in This Article: ] |
| 30. | Kanthan R, Saxena A, Kanthan SC. Goblet cell carcinoids of the appendix: immunophenotype and ultrastructural study. Arch Pathol Lab Med. 2001;125:386-390. [Cited in This Article: ] |
| 31. | Li CC, Hirowaka M, Qian ZR, Xu B, Sano T. Expression of E-cadherin, b-catenin, and Ki-67 in goblet cell carcinoids of the appendix: an immunohistochemical study with clinical correlation. Endocr Pathol. 2002;13:47-58. [Cited in This Article: ] |
| 32. | Modlin IM, Kidd M, Latich I, Zikusoka MN, Eick GN, Mane SM, Camp RL. Genetic differentiation of appendiceal tumor malignancy: a guide for the perplexed. Ann Surg. 2006;244:52-60. [Cited in This Article: ] |
| 33. | Edmonds P, Merino MJ, LiVolsi VA, Duray PH. Adenocarcinoid (mucinous carcinoid) of the appendix. Gastroenterology. 1984;86:302-309. [Cited in This Article: ] |
| 34. | Cooper PH, Warkel RL. Ultrastructure of the goblet cell type of adenocarcinoid of the appendix. Cancer. 1978;42:2687-2695. [Cited in This Article: ] |
| 35. | Byrn JC, Wang JL, Divino CM, Nguyen SQ, Warner RR. Management of goblet cell carcinoid. J Surg Oncol. 2006;94:396-402. [Cited in This Article: ] |
| 36. | Butler JA, Houshiar A, Lin F, Wilson SE. Goblet cell carcinoid of the appendix. Am J Surg. 1994;168:685-687. [Cited in This Article: ] |
| 37. | Hirschfield LS, Kahn LB, Winkler B, Bochner RZ, Gibstein AA. Adenocarcinoid of the appendix presenting as bilateral Krukenberg's tumor of the ovaries. Immunohistochemical and ultrastructural studies and literature review. Arch Pathol Lab Med. 1985;109:930-933. [Cited in This Article: ] |
| 38. | Toumpanakis C, Standish RA, Baishnab E, Winslet MC, Caplin ME. Goblet cell carcinoid tumors (adenocarcinoid) of the appendix. Dis Colon Rectum. 2007;50:315-322. [Cited in This Article: ] |
| 39. | Bak M, Asschenfeldt P. Adenocarcinoid of the vermiform appendix. A clinicopathologic study of 20 cases. Dis Colon Rectum. 1988;31:605-612. [Cited in This Article: ] |
| 40. | Haqqani MT, Williams G. Mucin producing carcinoid tumours of the vermiform appendix. J Clin Pathol. 1977;30:473-480. [Cited in This Article: ] |
| 41. | Chen V, Qizilbash AH. Goblet cell carcinoid tumor of the appendix. Report of five cases and review of the literature. Arch Pathol Lab Med. 1979;103:180-182. [Cited in This Article: ] |
| 42. | Olsson B, Ljungberg O. Adenocarcinoid of the vermiform appendix. Virchows Arch A Pathol Anat Histol. 1980;386:201-210. [Cited in This Article: ] |