Copyright
©The Author(s) 2025.
World J Gastrointest Oncol. Feb 15, 2025; 17(2): 100094
Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.100094
Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.100094
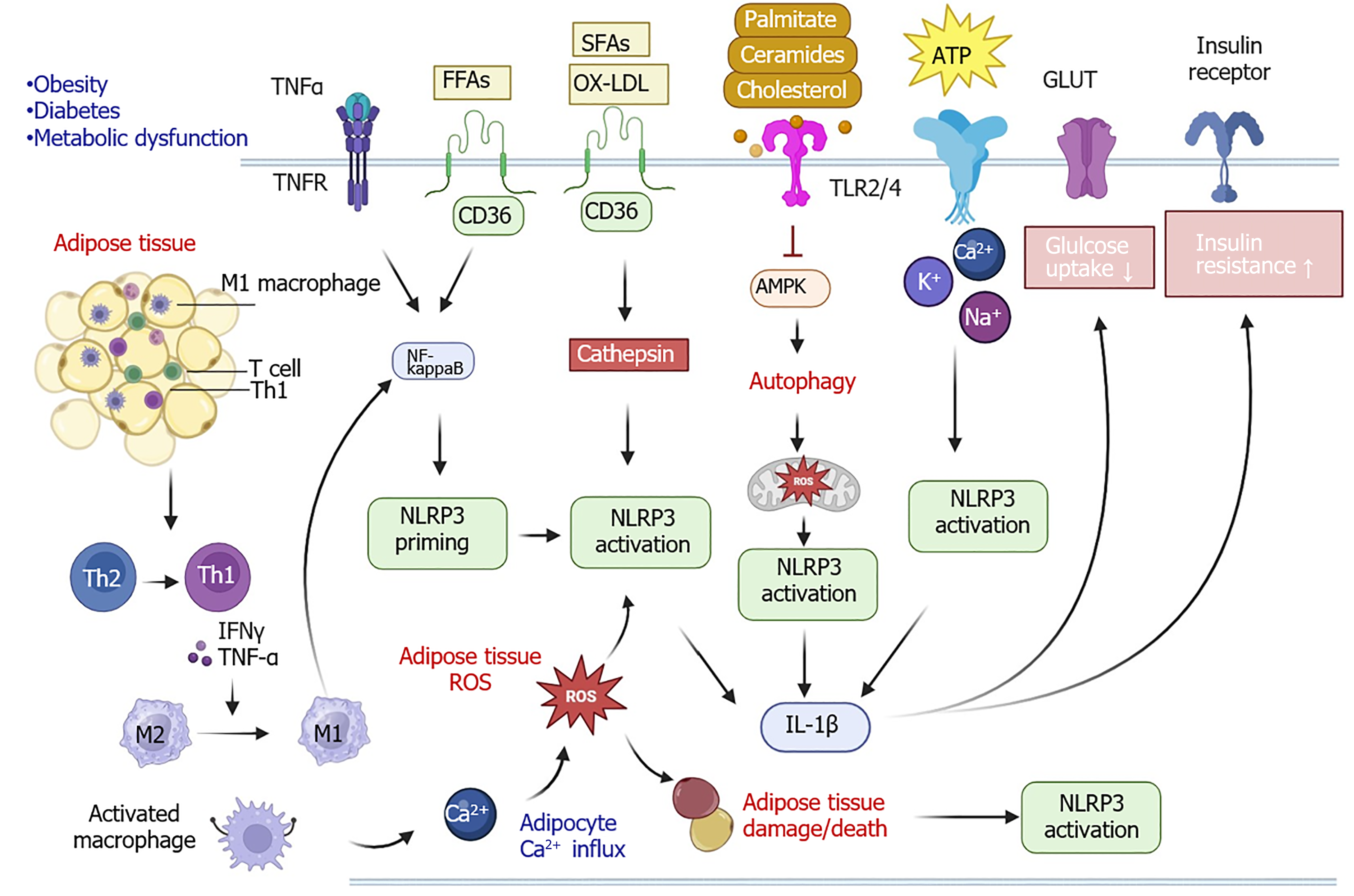
Figure 2 Molecular and cellular signaling pathways of nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 in obesity, diabetes, and other metabolic diseases.
In adipose tissue, interferon (IFN)-g and tumor necrosis factor (TNF)-α secreted from T helper (Th) 1 cells can promote macrophage differentiation from M2 to M1 phenotype. M1 macrophage infiltration results in the activation of nuclear factor kappa B (NF-kappaB) signaling pathway to induce nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3) priming. Simultaneously, calcium influx and reactive oxygen species (ROS) production can increase the recruitment of more macrophages. In addition, excessive levels of TNF-α and free fatty acids (FFAs) can activate the NF-kappaB signaling leading to NLRP3 priming and activation. Oxidized low-density lipoprotein (OX-LDL) and short-chain fatty acid (SFA) can bind with CD36 to accelerate the release of cathepsin causing the NLRP3 activation. Lipids such as palmitate, ceramides, and cholesterol can interact with toll-like receptor (TLR) 2/4 to inactivate the adenosine monophosphate-activated protein kinase (AMPK) pathway, resulting in mitochondrial ROS and NLRP3 activation. Additionally, adenosine triphosphate (ATP)-associated imbalance of Na+, Ca2+, and K+ also contributes to the activation of the NLRP3 inflammasome. NLRP3 activation raises the levels of interleukin (IL)-1β, which increases insulin resistance and decreases glucose uptake. The cartoons in this figure were prepared using Biorender (https://biorender.com). GLUT: Glucose transporter type; TNFR: Tumor necrosis factor receptor.
- Citation: Zhang CY, Liu S, Sui YX, Yang M. Nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 inflammasome: From action mechanism to therapeutic target in clinical trials. World J Gastrointest Oncol 2025; 17(2): 100094
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/100094.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.100094