Copyright
©The Author(s) 2024.
World J Gastrointest Oncol. Jul 15, 2024; 16(7): 3118-3157
Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3118
Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3118
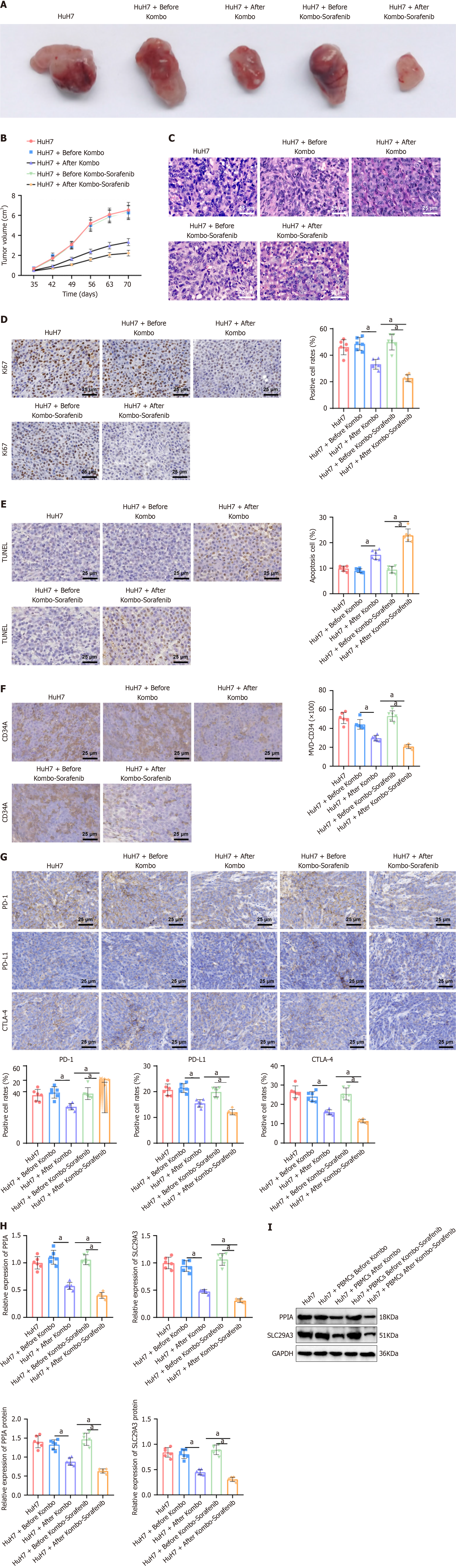
Figure 8 The effects of posttreatment with peripheral blood mononuclear cells on tumor formation and immune function in hepatocellular carcinoma mice were studied.
A: The influence of patient peripheral blood mononuclear cells (PBMCs) on the tumor growth of hepatocellular carcinoma (HCC) mice before and after treatment; B: Changes in relative tumor volume in each group of mice; C: Hematoxylin and eosin staining showing the histopathological morphology of tumor tissue, Bar = 400 ×; D: Immunohistochemical detection of the quantity of Ki-67 and analysis of the ratio of Ki-67-positive cells in the transplanted tumor, with arrows indicating tumor cell nuclei, Bar = 400 ×; E: TUNEL staining analysis of cell apoptosis and analysis of the ratio of transplanted tumor cell apoptosis, with arrows indicating tumor cell nuclei, Bar = 400 ×; F: Immunohistochemical detection of CD34 expression to assess tumor microvessel density, Bar = 400 ×; G: Immunohistochemical detection of immune checkpoints programmed cell death 1, programmed cell death ligand 1, and cytotoxic T lymphocyte-associated antigen-4, Bar = 400 ×; H and I: Real-time quantitative reverse transcription polymerase chain reaction (H) and Western blot (I) detection of the expression of peptidylprolyl isomerase A and solute carrier family 29 member 3 in tumor tissue. All groups included 6 mice per group, and the values are presented as the means ± SD. aP < 0.05 indicates a significant difference between the two groups (P < 0.05). PD-1: Programmed cell death 1; PD-L1: Programmed cell death ligand 1; CTLA-4: Cytotoxic T lymphocyte-associated antigen-4; PPIA: Peptidylprolyl isomerase A; SLC29A3: Solute carrier family 29 member 3.
- Citation: Cao Y, Li PP, Qiao BL, Li QW. Kombo knife combined with sorafenib in liver cancer treatment: Efficacy and safety under immune function influence. World J Gastrointest Oncol 2024; 16(7): 3118-3157
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3118.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3118