Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1154
Peer-review started: September 20, 2023
First decision: November 22, 2023
Revised: December 5, 2023
Accepted: February 2, 2024
Article in press: February 2, 2024
Published online: April 15, 2024
Minimally invasive surgery is a kind of surgical operation, which is performed by using professional surgical instruments and equipment to inactivate, resect, repair or reconstruct the pathological changes, deformities and wounds in human body through micro-trauma or micro-approach, in order to achieve the goal of treatment, its surgical effect is equivalent to the traditional open surgery, while avoiding the morbidity of conventional surgical wounds. In addition, it also has the advantages of less trauma, less blood loss during operation, less psychological burden and quick recovery on patients, and these minimally invasive techniques provide unique value for the examination and treatment of gastric cancer patients. Surgical minimally invasive surgical techniques have developed rapidly and offer numerous options for the treatment of early gastric cancer (EGC): endoscopic mucosal resection (EMR), underwater EMR (UEMR), endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (EFTR), endoscopic submu
Core Tip: In this article the authors provide an overview of the surgical forms of minimally invasive surgical treatment performed for early gastric cancer in recent years, adding newly popular surgical procedures such as band-assisted endoscopic mucosal resection (EMR), EMR with circumferential precutting, modified cap-assisted EMR, underwater EMR, ligation-assisted endoscopic full-thickness resection (EFTR), over-the-scope clip-assisted EFTR, no-touch EFTR, non-exposure simple suturing EFTR, exposed EFTR, and so on.
- Citation: Li CY, Wang YF, Luo LK, Yang XJ. Present situation of minimally invasive surgical treatment for early gastric cancer. World J Gastrointest Oncol 2024; 16(4): 1154-1165
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1154.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1154
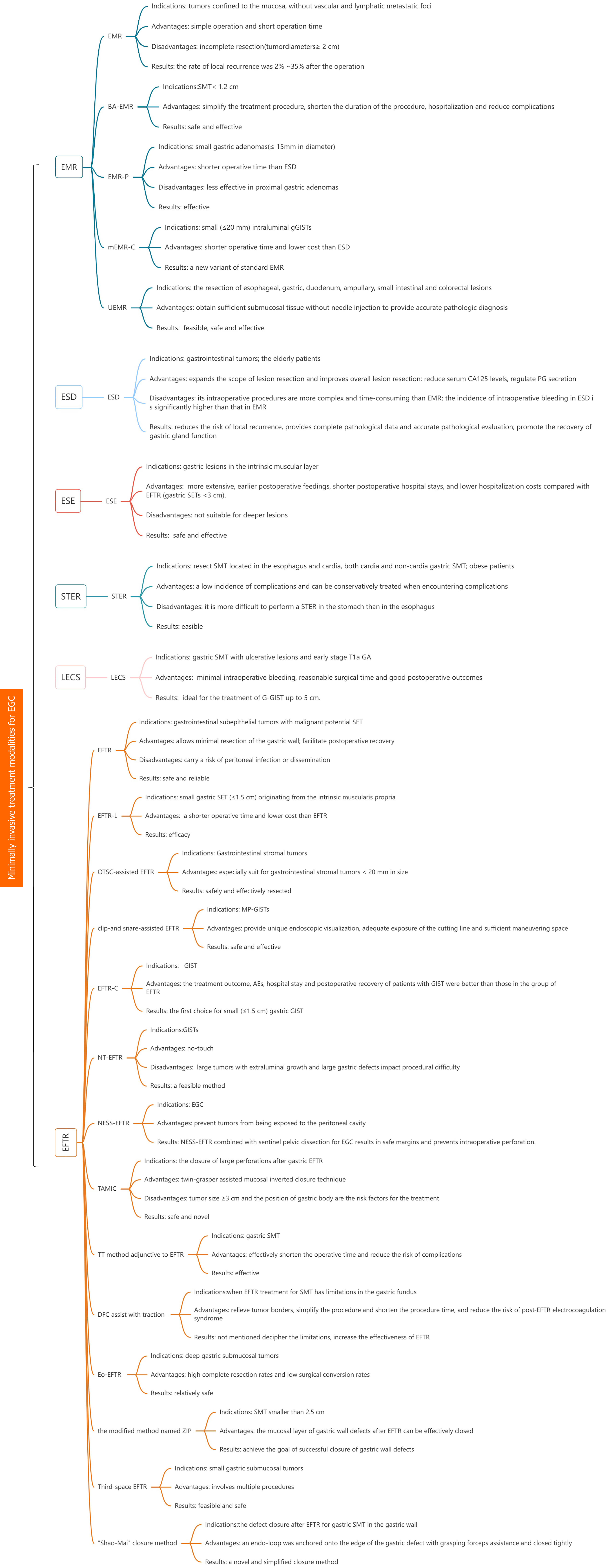
Minimally invasive surgery is a kind of surgical operation, which is performed by using professional surgical instruments and equipment to inactivate, resect, repair or reconstruct the pathological changes, deformities and wounds in human body through micro-trauma or micro-approach, in order to achieve the goal of treatment, its surgical effect is equivalent to the traditional open surgery, while avoiding the morbidity of conventional surgical wounds[1]. In addition, it also has the advantages of less trauma, less blood loss during operation, less psychological burden and quick recovery on patients, and these minimally invasive techniques provide unique value for the examination and treatment of gastric cancer (GC) patients. GC is one of the most common malignant tumors of digestive tract in the world. It is the fifth largest cancer after lung cancer, breast cancer, colorectal cancer and prostate cancer[2], in the meantime, GC is the third most common cause of cancer-related death due to the fact that its high mortality rate and its often advanced stage at the time of diagnosis[3]. Early intervention and treatment in the early stage of GC can effectively improve the prognosis of cancer patients. Surgical minimally invasive surgical techniques have developed rapidly and offer numerous options for the treatment of early GC (EGC), such as endoscopic mucosal resection (EMR), underwater EMR (UEMR), endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (EFTR), endoscopic submucosal excavation (ESE), submucosal tunnel endoscopic resection (STER), laparoscopic and endoscopic cooperative surgery (LECS) etc.; The purpose of this review is to discuss the utility of current minimally invasive surgical modalities in the management of EGC by weighing the benefits and limitations of minimally invasive surgical treatments for EGC. In addition, we aim to update the advances in minimally invasive treatment of EGC by considering the latest innovations in the field of minimally invasive surgical treatment of EGC, further defining any additional evidence of its role in minimally invasive treatment of EGC, complications, limitations of the technique and suggesting areas for further research (Figure 1). We present the following article in accordance with the narrative review reporting checklist.
In 1984, EMR was first reported in Japan for the treatment of EGC[4]. Due to its simple operation and short operation time, EMR was widely used in clinic. Early EMR was only suitable for early cancers with tumors confined to the mucosa, without vascular and lymphatic metastatic foci, or lesions that were locally not combined with ulcers, and tumors with diameters of more than 2 cm needed to be resected in several parts, slices and layers, which was easy to result in the incomplete resection of the lesions and residual cancerous tissues, and leaded to local recurrence after the operation, which was reported by Horiki et al[5]. The rate of recurrence was 2%-35%. In response to the limitations of conventional EMR (CEMR), several improved EMRs have emerged. A prospective study demonstrated that band-assisted EMR (BA-EMR) is an effective and safe method for small gastric fundus submucosal tumors (SMT) (< 1.2 cm)[6] , and that BA-EMR can simplify the treatment procedure, shorten the duration of the procedure, hospitalization, and reduce complications. It was found that EMR with circumferential precutting (EMR-P) was as effective as ESD in the treatment of small gastric adenomas(≤ 15 mm in diameter), with ESD having a significantly longer operative time than EMR-P, but EMR-P was less effective in proximal gastric adenomas[7]. Meng et al[8] reported that modified cap-assisted EMR (mEMR-C) is a new variant of standard EMR. The mEMR-C is the technique of choice for small (≤ 20 mm) intraluminal gastric gastrointestinal stromal tumors (gGISTs) with shorter operative time and lower cost than ESD. Meanwhile, on the basis of the EMR and saline solution-assisted snare or endoscopic cap-band mucosal resection technique (which allows for resection of lesions ≤ 14 mm in size in a single session) used by Karaca et al[9], the investigators modified the use of a beveled, clear-cap suction to resect small luminal gGISTs, which allows for suctioning of a larger volume of lesion, avoiding tumor remnants as much as possible, and increasing the rate of complete resection.
UEMR is a novel developed technique for the resection of esophageal, gastric, duodenum, ampullary, small intestinal and colorectal lesions[10].In gastric diseases, UEMR can also be used for establishing diagnosis of diffuse infiltrative GC[11]. CEMR can be used to diagnose invasive GC[12], but needle injection into hard tumor tissue is difficult and improper injection can make follow-up operation challenging and inconvenient. UEMR, in contrast, can obtain sufficient submucosal tissue without needle injection to provide accurate pathologic diagnosis. It has been shown that UEMR is feasible in gastric tumors of patients with FAP, particularly in elevated lesions and lesions ≤ 20 mm in diameter[13]. Kim et al[14] demonstrated that UEMR is a safe and effective treatment for upper gastrointestinal subepithelial tumors (SETs) originating from the deep mucosa and/or submucosa. Kim et al[15] reported the results of 4 cases of UEMR for benign mucosal tumors (< 15 mm in diameter) located in the pyloric ring, demonstrating that UEMR is an effective and safe method for the treatment of gastric pyloric ring tumors. During the UEMR procedure, water immersion allowed the lesion to float slightly and be easily identified, and then the whole resection was performed with a loop and an electrosurgical device. All procedures were operated quickly without adverse events (AEs).
The results of a multicenter randomized controlled trial indicated that UEMR, with a cutting plane depth comparable to that of CEMR, which can adequately resect the submucosal layer and is a feasible alternative for the histopathological evaluation of unpredictable submucosal invasive tumors[16].
ESD was first reported in Japan in 1988[17]. In contrast with EMR, ESD surgery expands the scope of lesion resection and improves overall lesion resection, which reduces the risk of local recurrence[18], provides complete pathological data and accurate pathological evaluation[19]. If there is a postoperative recurrence, it can be re-surgery to achieve the purpose of complete resection of the lesion and improve the cure rate. And ESD is suitable for the elderly patients[20], which has a high degree of safety and reliability. Research has shown that compared to EMR treatment, ESD can not only completely remove cancer lesions, but also reduce serum CA125 levels, regulate PG secretion, promote the recovery of gastric gland function, and reduce the risk of EGC recurrence[21]. However, its intraoperative procedures are more complex and time-consuming than EMR[22], with a larger resection range of tissue and a wider and deeper ulcer base. Therefore, caution should be exercised to reduce complications such as bleeding and perforation. Due to longer surgical time and greater invasiveness, the incidence of intraoperative bleeding in ESD is significantly higher than that in EMR. The review first defines post-ESD bleeding and elaborates on its management, including methods for coagulation of potential bleeding points during surgery, lesion closure, lesion shielding, and the application of gastric acid secretion inhibitors[23].
A North American study mentioned[24] that in Asia, ESD has been proven to be superior to EMR; This large multicenter prospective trial evaluated ESD in North America, demonstrating that ESD can be safely and effectively performed with a low postoperative recurrence rate, further supporting the implementation of ESD treatment for gastrointestinal tumors.
The EFTR technique was first introduced in Japan in 1998[25], and subsequently the same team reported that it is safe and reliable for completely closed early gastrointestinal malignancies tumors[26]. The use of commercially available EMR kit devices to assist EFTR has been shown to be a safe and feasible approach for endoscopic resection of gastrointestinal SETs with malignant potential SET[27]. Mucosal resection and limited submucosal dissection to preserve the mucosa prior to tumor resection in SET patients may facilitate postoperative recovery. Ligation-assisted EFTR has been demonstrated efficacy in treating small gastric SETs (≤ 1.5 cm) originating from the intrinsic muscularis propria, with a shorter operative time and lower cost than EFTR[28].
gGISTs can be safely and effectively resected by the over-the-scope clip-assisted EFTR, especially for gGISTs < 20 mm in size[29]. A Novel approach to clip-and snare-assisted EFTR (also named as m-EFTR or chen-EFTR) safely and effectively resects muscularis propria layer GISTs by providing unique endoscopic visualization, adequate exposure of the cutting line and sufficient maneuvering space[30]. It has been shown that[31] double-curved endoscope has an advantage over single-curved endoscopes in the duration of EFTR surgery in gGISTs, especially in the fundus of the stomach. Yang et al[32] found that the treatment outcome, AEs, hospital stay and postoperative recovery of patients with GIST in the group of cap-assisted EFTR were better than those in the group of EFTR, which may be the first choice for small (≤ 1.5 cm) gastric GIST. The new no-touch EFTR technique developed by Chen et al[33] is a feasible approach for GIST resection and holds promise for complete radical resection. The growth of large extra-cavity tumor is one of the factors affecting the difficulty of surgery. EFTR locates the tumor endoscopically and carries the risk of peritoneal infection or dissemination while maximizing the resection of the gastric wall. In response to this question, a new EFTR[34] technique has been devised: silicone sheets and gauze are attached to the plasma membrane of the intact porcine stomach using a fibrinogen-thrombin solution to prevent gastric juices from escaping before proceeding to subsequent surgical steps. The experimental results show that the time required to perform a seromuscular incision is significantly shorter with the new EFTR technique, which avoids exposure of the tumor to the peritoneal cavity while incising all layers of the stomach, and that gastric collapse can be prevented using this technique. Non-exposure simple suturing EFTR (NESS-EFTR) can also prevent tumors from being exposed to the peritoneal cavity. Studies have shown[35] that NESS-EFTR combined with sentinel pelvic dissection for EGC results in safe margins and prevents intraoperative perforation. The closure of large perforations after gastric EFTR can be achieved by the safe and novel twin-grasper assisted mucosal inverted closure technique[36]. The study has showed[37] that the tumor size ≥ 3 cm and the position of gastric body are the risk factors for the treatment of SMT with EFTR. When intraoperative tumor exposure is suboptimal, the thread-traction method adjunctive to EFTR for gastric SMT can effectively shorten the operative time and reduce the risk of complications[38]. Exposed EFTR is relatively safe with high complete resection rates and low surgical conversion rates in the treatment of deep gastric SMT[39]. When EFTR treatment for SMT has limitations in the gastric fundus, the use of dental floss and a hemoclip to assist with traction can decipher the limitations, increase the effectiveness of EFTR, relieve tumor borders, simplify the procedure and shorten the procedure time, and reduce the risk of post-EFTR electrocoagulation syndrome[40,41]. To achieve the goal of successful closure of gastric wall defects, for SMT smaller than 2.5 cm, the mucosal layer of gastric wall defects after EFTR can be effectively closed by the modified method named ZIP[42]; Third-space EFTR is one of the minimally invasive endoscopic options for the treatment of small gastric SMT, which involves multiple procedures such as circumferential mucosal incisions, proximal submucosal tunneling, peripheral mucosal endoscopic suturing, circumferential serosal myotomy of the submucosal tunneling, transoral retrieval, and closure of the tunneling entrance site[43]. It is feasible and safe. The endoscopic "Shao-Mai" closure method is a novel and simplified closure method[44]: after successful resection of the tumor via the EFTR, a grasping forceps-assisted internal loop ligation device is used to secure the lining to the edge of the gastric defect and close it tightly. The Koreans[45] have also added a sentinel lymph node drainage area clearance at the tumor site to the EFTR procedure, which obtained a good oncologic outcome while providing maximum protection to the patient's postoperative gastric function.
ESE evolved from ESD technology[46]. ESE involves a longitudinal or circular incision in the mucosa overlying the lesion to resect the lesion, which is more extensive but not suitable for deeper lesions. The literature suggests that the ESE technique is safe and effective for gastric[47,48] lesions in the intrinsic muscular layer.
When treating gastric SETs (< 3 cm), ESE resulted in earlier postoperative feedings, shorter postoperative hospital stays, and lower hospitalization costs compared with EFTR[49]. Compared with STER, the removal time of ESE was shorter but the wound closure time was longer when treating upper gastroenterology SMT originating from the muscularis propria layer[50,51], with no significant difference in total operative time. The operation time of ESE surgery is shorter than that of STER in the treatment of cardiac SMT[52], and the intraoperative risk factor is the irregular tumor shape.
STER was originally used to resect SMT located in the esophagus and cardia, and it is feasible to treat both cardia and non-cardia gastric SMT with comparable efficacy[53]. Because of differences in anatomical and physiological characteristics, it is more difficult to perform a STER in the stomach than in the esophagus. A meta-analysis[54] evaluating the results showed that STER treatment for gastric SMT has a low incidence of complications and can be conservatively treated when encountering complications. A study showed[55] a low incidence of short-term complications in large SMT originating from the muscularis propria of esophagus and gastric cardia, with the most common complication during or after surgery being perforation. A study with a follow-up time of over 1 year showed[56] that STER has a clear therapeutic effect on upper gastrointestinal SMTs, but the incidence of AE is not low. Conservative treatment can be used when AE occurs. STER is a safe and effective procedure for resecting SETs of the gastrointestinal tract[57]. STER is also safe and effective for obese patients awaiting surgical treatment and does not interfere with bariatric surgery[58]. Independent risk factors for postoperative complications after endoscopic treatment of subepithelial lesions were lesion diameter greater over 4 cm and operative time greater than 2 h[59]. All surgical treatments require a high degree of vigilance against the occurrence of postoperative complications.
It was first performed LECS by Hiki et al[60] in 2008 for dissection of gastric SMT (such as GIST) with minimal intraoperative bleeding, reasonable surgical time and good postoperative outcomes. In 2012, the medical team achieved good results in treating EGC with a wide range of lesions with LECS[61]. Initially, the indication for classical LECS was gastric SMT without ulcerative lesions[62]. The indication for LECS was expanded to include gastric SMT with ulcerative lesions and early stage T1a GC without lymph node metastasis[63], with the development and advancement of the technology, a number of improved LECS procedures have gradually emerged, expanding the indications for LECS. The inverted LECS technique involves inverting the tumor into the gastric cavity during surgery, which can avoid contamination with gastric juices as well as direct contact between the surrounding tissues and the tumor[64], removing the tumor and placing it into the gastric cavity, Finally, the tumor was removed from the mouth. A case of successful treatment of GIST near the pyloric ring using the inverted LECS demonstrated that LECS can preserve the function of the cardia and pylorus by minimal resection without blocking the passage or stasis[64]. The CLEAN-NET procedure preserves the mucosa and prevents the flow of gastric contents into the peritoneal cavity[65]; CLEAN-NET can be used in conjunction with lymph node dissection for further treatment of the disease after completion of a total gastric wall resection. CLEAN-NET has been found to be safe and useful in the treatment of gastric GIST with ulceration[66]. Nineteen patients treated with CLEAN-NET were studied[67], all of whom had their tumors removed as a whole with no intraoperative ruptures. It was performed safely within an average operative time of 105.4 minutes and the postoperative course was uneventful. A patient with gastric heterotopic inverted polyp (GHIP)[68], which was difficult to diagnose accurately due to the location of the polyp and difficult to resect the tissue. It was diagnosed and treated with a modified CLEAN-NET. The good postoperative results show that the modified CLEAN-NET can treat SMT while avoiding gastric metaplasia and tumor dissemination, as in the case of GHIP with a central dimple. The researchers performed closed LECS in three cases of EGC after ESD failure[69], by which gastric tumors can be accurately removed without exposing tumor cells to the abdominal cavity. Closed LECS is less invasive in the treatment of EGC.
Surgical minimally invasive procedures are increasingly being used for the treatment and resection of EGC, thanks to their flexibility in removing tumors in anatomically challenging areas while providing precision to minimize the removal of undiseased tissue margins. Within this already widely used technique, researchers have explored a number of different improved and innovative surgical approaches based on tumor factors and surgeon selection, allowing for dynamic optimization of tailoring the appropriate surgical technique for different patients in different situations (Table 1). EMR, EFTR and LECS are a few of the more widely used surgical techniques for treating early stage, and the most derived and innovative measures based on them are likely to become more widespread and indispensable in the future for treating EGC.
| Initial | Upgrade | Indications | Advantages | Disadvantages | Results |
| EMR | EMR | Tumors confined to the mucosa, without vascular and lymphatic metastatic foci | Simple operation and short operation time | Incomplete resection (tumor diameters ≥ 2 cm) | The rate of local recurrence was 2%-35% after the operation[5] |
| BA-EMR | SMT (< 1.2 cm)[6] | Simplify the treatment procedure, shorten the duration of the procedure, hospitalization and reduce complications | Not mentioned | Safe and effective | |
| EMR-P | Small gastric adenomas (≤ 15 mm in diameter) | Shorter operative time than ESD | Less effective in proximal gastric adenomas[7] | Effective | |
| mEMR-C | Small (≤ 20 mm) intraluminal gGISTs | Shorter operative time and lower cost than ESD | Not mentioned | A new variant of standard EMR | |
| UEMR | UEMR | The resection of esophageal, gastric, duodenum, ampullary, small intestinal and colorectal lesions[10] | Obtain sufficient submucosal tissue without needle injection to provide accurate pathologic diagnosis | Not mentioned | Feasible, safe and effective |
| ESD | ESD | Gastrointestinal tumors; the elderly patients[20] | Expands the scope of lesion resection and improves overall lesion resection; reduce serum CA125 levels, regulate PG secretion | Its intraoperative procedures are more complex and time-consuming than EMR[22]; the incidence of intraoperative bleeding in ESD is significantly higher than that in EMR | Reduces the risk of local recurrence[18], provides complete pathological data and accurate pathological evaluation[19]; promote the recovery of gastric gland function[21] |
| EFTR | EFTR | Gastrointestinal subepithelial tumors with malignant potential SET[27] | Allows minimal resection of the gastric wall; facilitate postoperative recovery | Carry a risk of peritoneal infection or dissemination | Safe and reliable |
| EFTR-L | Small gastric SET (≤ 1.5 cm) originating from the intrinsic muscularis propria | A shorter operative time and lower cost than EFTR[28] | Not mentioned | Efficacy | |
| OTSC-assisted EFTR | Gastrointestinal stromal tumors | Especially suit for gastrointestinal stromal tumors < 20 mm in size[29] | Not mentioned | Safely and effectively resected | |
| Clip-and snare-assisted EFTR | MP-GISTs | Provide unique endoscopic visualization, adequate exposure of the cutting line and sufficient maneuvering space[30] | Not mentioned | Safe and effective | |
| EFTR-C | GIST | The treatment outcome, AEs, hospital stay and postoperative recovery of patients with GIST were better than those in the group of EFTR | Not mentioned | The first choice for small (≤ 1.5 cm) gastric GIST | |
| NT-EFTR | GISTs | No-touch | Large tumors with extraluminal growth and large gastric defects impact procedural difficulty | A feasible method | |
| NESS-EFTR | EGC | Prevent tumors from being exposed to the peritoneal cavity | Not mentioned | NESS-EFTR combined with sentinel pelvic dissection for EGC results in safe margins and prevents intraoperative perforation | |
| TAMIC | The closure of large perforations after gastric EFTR | Twin-grasper assisted mucosal inverted closure technique[36] | Tumor size ≥ 3 cm and the position of gastric body are the risk factors for the treatment | Safe and novel | |
| TT method adjunctive to EFTR | Gastric SMT | Effectively shorten the operative time and reduce the risk of complications[38] | Not mentioned | Effective | |
| Eo-EFTR | Deep gastric submucosal tumors | High complete resection rates and low surgical conversion rates | Not mentioned | Relatively safe | |
| DFC assist with traction | When EFTR treatment for SMT has limitations in the gastric fundus | Relieve tumor borders, simplify the procedure and shorten the procedure time, and reduce the risk of post-EFTR electrocoagulation syndrome[40,41] | Not mentioned | Decipher the limitations, increase the effectiveness of EFTR | |
| The modified method named ZIP | SMT smaller than 2.5 cm | The mucosal layer of gastric wall defects after EFTR can be effectively closed | Not mentioned | Achieve the goal of successful closure of gastric wall defects | |
| Third-space EFTR | Small gastric submucosal tumors | Involves multiple procedures[43] | Not mentioned | Feasible and safe | |
| "Shao-Mai" closure method | The defect closure after EFTR for gastric SMT in the gastric wall | An endo-loop was anchored onto the edge of the gastric defect with grasping forceps assistance and closed tightly | Not mentioned | A novel and simplified closure method[44] | |
| ESE | ESE | Gastric lesions in the intrinsic muscular layer | More extensive, earlier postoperative feedings, shorter postoperative hospital stays, and lower hospitalization costs compared with EFTR[49] (gastric SETs < 3 cm) | Not suitable for deeper lesions | Safe and effective |
| STER | STER | Resect SMT located in the esophagus and cardia, both cardia and non-cardia gastric SMT; obese patients | A low incidence of complications and can be conservatively treated when encountering complications | It is more difficult to perform a STER in the stomach than in the esophagus | Easible |
| LECS | LECS | Gastric SMT with ulcerative lesions and early stage T1a GC[63,64] | Minimal intraoperative bleeding, reasonable surgical time and good postoperative outcomes | Not mentioned | Ideal for the treatment of G-GIST up to 5 cm |
The authors offered technical support and materials used for experiments.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kinami S, Japan; Yildirim M, Turkey S-Editor: Gao CC L-Editor: A P-Editor: Yu HG
| 1. | Jaffray B. Minimally invasive surgery. Arch Dis Child. 2005;90:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2020: stomach cancer fact sheet. (2020). [cited 17 December 2020]. In: World Health Organization [Internet]. Available from: https://www.bing.com/ck/a?!&&p=35d79a372e8143d7JmltdHM9MTcwNjQ4NjQwMCZpZ3VpZD0zZjkxYTRjOC01MDY1LTY1YTktMDFiOC1iNjI1NTE0YjY0M2MmaW5zaWQ9NTE3Nw&ptn=3&ver=2&hsh=3&fclid=3f91a4c8-5065-65a9-01b8-b625514b643c&u=a1aHR0cHM6Ly9nY28uaWFyYy5mci90b2RheS9kYXRhL2ZhY3RzaGVldHMvY2FuY2Vycy83LVN0b21hY2gtZmFjdC1zaGVldC5wZGY&ntb=1. [Cited in This Article: ] |
| 3. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1150] [Cited by in F6Publishing: 1812] [Article Influence: 453.0] [Reference Citation Analysis (0)] |
| 4. | Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 289] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Fukuda K, Fujita Y, Ninomiya K, Tano S, Katurahara M, Tanaka K, Gabazza EC, Takei Y. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc. 2012;26:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Pan W, Shi D. Band-assisted endoscopic mucosal resection for small (≤ 1.5 cm) submucosal tumors originating from the muscularis propria in the gastric fundus: a prospective study. Surg Endosc. 2023;37:1806-1812. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 7. | Cho JH, Shin CM, Yoon H, Park YS, Kim N, Lee DH. Comparison of endoscopic treatments for small gastric adenomas. Surg Endosc. 2022;36:3920-3931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Meng R, Ni M, Ren W, Zhou T, Zhang X, Yan P, Ding X, Xu G, Lv Y, Zou X, Zhou L, Wang L. Comparison of Modified Cap-Assisted Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection in Treating Intraluminal Gastric Gastrointestinal Stromal Tumor (≤20 mm). Clin Transl Gastroenterol. 2023;14:e00589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 9. | Karaca C, Daglilar ES, Soyer OM, Gulluoglu M, Brugge WR. Endoscopic submucosal resection of gastric subepithelial lesions smaller than 20 mm: a comparison of saline solution-assisted snare and cap band mucosectomy techniques. Gastrointest Endosc. 2017;85:956-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Takeuchi Y, Shichijo S, Uedo N, Ishihara R. Underwater endoscopic mucosal resection for colorectal lesions: Can it be an "Underwater" revolution? DEN Open. 2022;2:e84. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Hamada K, Uedo N, Hanaoka N, Ishihara R, Oh Y. Gastrointestinal: Endoscopic mucosal resection for diagnosis of infiltrating gastric cancer: A case report. J Gastroenterol Hepatol. 2016;31:1670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Kawakami Y, Shichijo S, Takeuchi Y, Kubo C, Omori T, Uedo N. Underwater EMR for the diagnosis of diffuse infiltrative gastric cancer. VideoGIE. 2023;8:68-69. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 13. | Shimamoto Y, Takeuchi Y, Ishiguro S, Nakatsuka SI, Yunokizaki H, Ezoe Y, Matsuno K, Nakahira H, Shichijo S, Maekawa A, Kanesaka T, Yamamoto S, Higashino K, Uedo N, Ishihara R, Ishikawa H. Feasibility of underwater endoscopic mucosal resection for endoscopic management of gastric neoplasms in patients with familial adenomatous polyposis. Surg Endosc. 2023;37:6877-6884. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 14. | Kim SJ, Kim TU, Choi CW, Kim HW, Park SB, Ryu DG. Underwater endoscopic mucosal resection of upper gastrointestinal subepithelial tumors: A case series pilot study (with video). Medicine (Baltimore). 2022;101:e31072. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 15. | Kim DH, Park SY, Park CH, Kim HS, Choi SK. Underwater endoscopic mucosal resection for neoplasms in the pyloric ring of the stomach: Four case reports. World J Clin Cases. 2020;8:3050-3056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Matsueda K, Takeuchi Y, Kitamura M, Yamashina T, Akasaka T, Iwatsubo T, Nakatani Y, Akamatsu T, Kawamura T, Fujii S, Kusaka T, Shimokawa T, Uedo N. Depth of the cutting plane with underwater and conventional endoscopic mucosal resection: Post-hoc analysis of a randomized study. J Gastroenterol Hepatol. 2022;37:741-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 250] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 20. | Tokioka S, Umegaki E, Murano M, Takeuchi N, Takeuchi T, Kawakami K, Yoda Y, Kojima Y, Higuchi K. Utility and problems of endoscopic submucosal dissection for early gastric cancer in elderly patients. J Gastroenterol Hepatol. 2012;27 Suppl 3:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Lei HJ, Pan J, Liu J, Yao Y, Feng L. Comparison of therapeutic effects between ESD and EMR in early gastric cancer patients and their effects on serum CA125 and pepsinogen levels. Linchuang Xiaohuabing Zazhi. 2023;35:203-206. [Cited in This Article: ] |
| 22. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 529] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 23. | Liu L, Liu H, Feng Z. A narrative review of postoperative bleeding in patients with gastric cancer treated with endoscopic submucosal dissection. J Gastrointest Oncol. 2022;13:413-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Draganov PV, Aihara H, Karasik MS, Ngamruengphong S, Aadam AA, Othman MO, Sharma N, Grimm IS, Rostom A, Elmunzer BJ, Jawaid SA, Westerveld D, Perbtani YB, Hoffman BJ, Schlachterman A, Siegel A, Coman RM, Wang AY, Yang D. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology. 2021;160:2317-2327.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 25. | Suzuki H, Okuwaki S, Ikeda K. Endoscopic full-thickness resection (EFTR) and waterproof defect closure (ENDC) for improvement of curability and safety in endoscopic treatment of early gastrointestinal malignancies. Prog Dig Endosc. 1998;52:49-53. [Cited in This Article: ] |
| 26. | Suzuki H, Ikeda K. Endoscopic mucosal resection and full thickness resection with complete defect closure for early gastrointestinal malignancies. Endoscopy. 2001;33:437-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Li LY, Li BW, Mekaroonkamol P, Chen HM, Shen SS, Luo H, Dacha S, Xue Y, Cristofaro S, Keilin S, Willingham F, Cai Q. Mucosectomy device-assisted endoscopic resection of gastric subepithelial lesions. J Dig Dis. 2020;21:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Gu L, Wu Y, Yi J, Ouyang M, Liu X. Comparison of endoscopic full-thickness resection and ligation-assisted endoscopic full-thickness resection for small (≤ 1.5 cm) gastric subepithelial tumors originating from muscularis propria. Surg Endosc. 2023;37:3796-3806. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 29. | Wang W, Liu CX, Niu Q, Wang AL, Shi N, Ma FZ, Hu YB. OTSC assisted EFTR for the treatment of GIST: 40 cases analysis. Minim Invasive Ther Allied Technol. 2022;31:238-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 30. | Tian X, Shi B, Chen WQ. Modified endoscopic full-thickness resection of gastric stromal tumor originating from the muscularis propria layer. J Gastrointest Oncol. 2020;11:461-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Liu L, Xu X, Wang Q, Feng Y, Lu F, Tian Q, Shi D, Li R, Chen W. An evaluation of the use of double-curved endoscopes for gastric gastrointestinal stromal tumors. Minim Invasive Ther Allied Technol. 2023;32:112-118. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 32. | Yang J, Ni M, Jiang J, Ren X, Zhu T, Cao S, Hassan S, Lv Y, Zhang X, Wei Y, Wang L, Xu G. Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤1.5 cm) gastric GI stromal tumors. Gastrointest Endosc. 2022;95:660-670.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Chen T, Zhang YW, Lian JJ, Zhang HB, Xu AP, Li F, Yan XH, Duan BS, Zhao ZY, Chu Y, Shen L, Cao J, Zhang L, Zheng L, Chu SG, Xu MD. No-touch endoscopic full-thickness resection technique for gastric gastrointestinal stromal tumors. Endoscopy. 2023;55:557-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 34. | Kitakata H, Itoh T, Kinami S, Kawaura K, Hamada K, Azukisawa S, Kobayashi R, Kamai J, Kosaka T. Sealed endoscopic full-thickness resection for gastric cancer: a pilot study in an ex vivo and in vivo porcine model. Endosc Int Open. 2019;7:E36-E42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Eom BW, Kim CG, Kook MC, Yoon HM, Ryu KW, Kim YW, Rho JY, Kim YI, Lee JY, Choi IJ. Non-exposure Simple Suturing Endoscopic Full-thickness Resection with Sentinel Basin Dissection in Patients with Early Gastric Cancer: the SENORITA 3 Pilot Study. J Gastric Cancer. 2020;20:245-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Cai Q, Fu H, Zhang L, Shen M, Yi S, Xie R, Lan W, Dong W, Chen X, Zhang J, Hou X, He Y, Yang D. Twin-grasper assisted mucosal inverted closure achieves complete healing of large perforations after gastric endoscopic full-thickness resection. Dig Endosc. 2023;35:736-744. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 37. | Jian G, Tan L, Wang H, Lv L, Wang X, Qi X, Le M, Tan Y, Liu D. Factors that predict the technical difficulty during endoscopic full-thickness resection of a gastric submucosal tumor. Rev Esp Enferm Dig. 2021;113:35-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Li J, Meng Y, Ye S, Wang P, Liu F. Usefulness of the thread-traction method in endoscopic full-thickness resection for gastric submucosal tumor: a comparative study. Surg Endosc. 2019;33:2880-2885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Granata A, Martino A, Ligresti D, Tuzzolino F, Lombardi G, Traina M. Exposed endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors: A systematic review and pooled analysis. Dig Liver Dis. 2022;54:729-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Li B, Shi Q, Qi ZP, Yao LQ, Xu MD, Lv ZT, Yalikong A, Cai SL, Sun D, Zhou PH, Zhong YS. The efficacy of dental floss and a hemoclip as a traction method for the endoscopic full-thickness resection of submucosal tumors in the gastric fundus. Surg Endosc. 2019;33:3864-3873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Shi Q, Li B, Qi ZP, Yao LQ, Xu MD, Cai SL, Sun D, Zhou PH, Zhong YS. Clinical Values of Dental Floss Traction Assistance in Endoscopic Full-Thickness Resection for Submucosal Tumors Originating from the Muscularis Propria Layer in the Gastric Fundus. J Laparoendosc Adv Surg Tech A. 2018;28:1261-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Li Y, Cui Z, Yu J, Bao X, Wang S. Do we need to conduct full-thickness closure after endoscopic full-thickness resection of gastric submucosal tumors? Turk J Gastroenterol. 2020;31:942-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Goto O, Sasaki M, Akimoto T, Tatsuguchi A, Kaise M, Iwakiri K, Yahagi N. Feasibility and safety of third-space endoscopic full-thickness resection in ex vivo and in vivo porcine models. Endosc Int Open. 2019;7:E471-E476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | He J, Chen BS, Zhou PH, Zhong YS, Chen WF, Zhang YQ, Li QL, Hu JW. [A novel and simplified closure method for defect closure after endoscopic full-thickness resection of gastric submucosal tumors: short-term outcomes of "Shao-Mai" closure method]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:183-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 45. | Hur H, Lim SG, Byun C, Kang JK, Shin SJ, Lee KM, Kim JH, Cho YK, Han SU. Laparoscopy-assisted endoscopic full-thickness resection with basin lymphadenectomy based on sentinel lymph nodes for early gastric cancer. J Am Coll Surg. 2014;219:e29-e37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Kim SY, Kim KO. Endoscopic Treatment of Subepithelial Tumors. Clin Endosc. 2018;51:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Wang S, Shen L. Efficacy of Endoscopic Submucosal Excavation for Gastrointestinal Stromal Tumors in the Cardia. Surg Laparosc Endosc Percutan Tech. 2016;26:493-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Wang Y, Li Y, Luo H, Yu H. [Efficacy analysis of endoscopic submucosal excavation for gastric gastrointestinal stromal tumors]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:352-355. [PubMed] [Cited in This Article: ] |
| 49. | Li DM, Ren LL, Jiang YP. Long-term Outcomes of Endoscopic Resection for Gastric Subepithelial Tumors. Surg Laparosc Endosc Percutan Tech. 2020;30:187-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Tian XL, Huang YH, Yao W, Li Y, Lu JJ. [Comparative treatment analysis of upper gastroenterology submucosal tumors originating from muscularis propria layer: submucosal tunneling endoscopic resection vs endoscopic submucosal excavation]. Beijing Da Xue Xue Bao Yi Xue Ban. 2019;51:171-176. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 51. | Ponte Neto FL, de Moura DTH, Sagae VMT, Ribeiro IB, Mancini FC, Boghossian MB, McCarty TR, Miyajima NT, Ide E, Bernardo WM, de Moura EGH. Endoscopic resection of esophageal and gastric submucosal tumors from the muscularis propria layer: submucosal tunneling endoscopic resection vs endoscopic submucosal excavation: A systematic review and meta-analysis. Surg Endosc. 2021;35:6413-6426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Du C, Chai N, Linghu E, Gao Y, Li Z, Li L, Zhai Y, Lu Z, Meng J, Tang P. Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection vs endoscopic submucosal excavation. Surg Endosc. 2018;32:4543-4551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Tan Y, Zhou B, Zhang S, Deng F, Li R, Gao S, Huo J, Liu D. Submucosal Tunneling Endoscopic Resection for Gastric Submucosal Tumors: a Comparison Between Cardia and Non-cardia Location. J Gastrointest Surg. 2019;23:2129-2135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Cao B, Lu J, Tan Y, Liu D. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021;113:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Wang Z, Zheng Z, Wang T, Wang X, Cao Y, Wang Y, Wang B. Submucosal tunneling endoscopic resection of large submucosal tumors originating from the muscularis propria layer in the esophagus and gastric cardia. Z Gastroenterol. 2019;57:952-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Peng W, Tan S, Huang S, Ren Y, Li H, Peng Y, Fu X, Tang X. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors with more than 1-year' follow-up: a systematic review and meta-analysis. Scand J Gastroenterol. 2019;54:397-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Nabi Z, Ramchandani M, Sayyed M, Darisetty S, Kotla R, Rao GV, Reddy DN. Outcomes of submucosal tunneling endoscopic resection in upper gastrointestinal sub-epithelial tumors. Indian J Gastroenterol. 2019;38:509-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Donatelli G, Cereatti F, Dumont JL, Trelles N, Lainas P, Dammaro C, Tranchart H, Pacini F, Arienzo R, Chevalier JM, Danan D, Catheline JM, Dagher I. Submucosal Tunnel Endoscopic Resection of Gastric Lesion Before Obesity Surgery: a Case Series. Obes Surg. 2020;30:4636-4642. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 59. | Li P, Li S, Liu S, Zhang D. Risk factors for complications of therapeutic endoscopy for upper gastrointestinal subepithelial lesions. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46:278-282. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 60. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 61. | Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 62. | Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 63. | Hiki N, Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann Gastroenterol Surg. 2019;3:239-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Watanabe M, Doita S, Minagi H, Miyake E, Hatono M, Ogawa T, Kimura Y, Taniguchi F, Arata T, Katsuda K, Tanakaya K, Aoki H. [A Case of Inverted LECS for GIST Near the Pylorus Ring]. Gan To Kagaku Ryoho. 2022;49:1449-1451. [PubMed] [Cited in This Article: ] |
| 65. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. 2012;21:129-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 66. | Nabeshima K, Tomioku M, Nakamura K, Yasuda S. Combination of Laparoscopic and Endoscopic Approaches to Neoplasia with Non-exposure Technique (CLEAN-NET) for GIST with Ulceration. Tokai J Exp Clin Med. 2015;40:115-119. [PubMed] [Cited in This Article: ] |
| 67. | Kanehira E, Kanehira AK, Tanida T, Takahashi K, Obana Y, Sasaki K. CLEAN-NET: a modified laparoendoscopic wedge resection of the stomach to minimize the sacrifice of innocent gastric wall. Surg Endosc. 2020;34:290-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Hayase S, Sakuma M, Chida S, Saito M, Ami H, Koyama Y, Ohki S, Kono K. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) using a modified combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique (modified CLEAN-NET): a case report. Surg Case Rep. 2020;6:200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Saito H, Nishimura A, Sakimura Y, Tawara H, Hayashi K, Kato K, Tsuji T, Yamamoto D, Kitamura H, Kadoya S, Bando H. Closed laparoscopic and endoscopic cooperative surgery for early gastric cancer with difficulty in endoscopic submucosal dissection: a report of three cases. Surg Case Rep. 2020;6:235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |