Copyright
©The Author(s) 2022.
World J Gastrointest Oncol. Sep 15, 2022; 14(9): 1665-1674
Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1665
Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1665
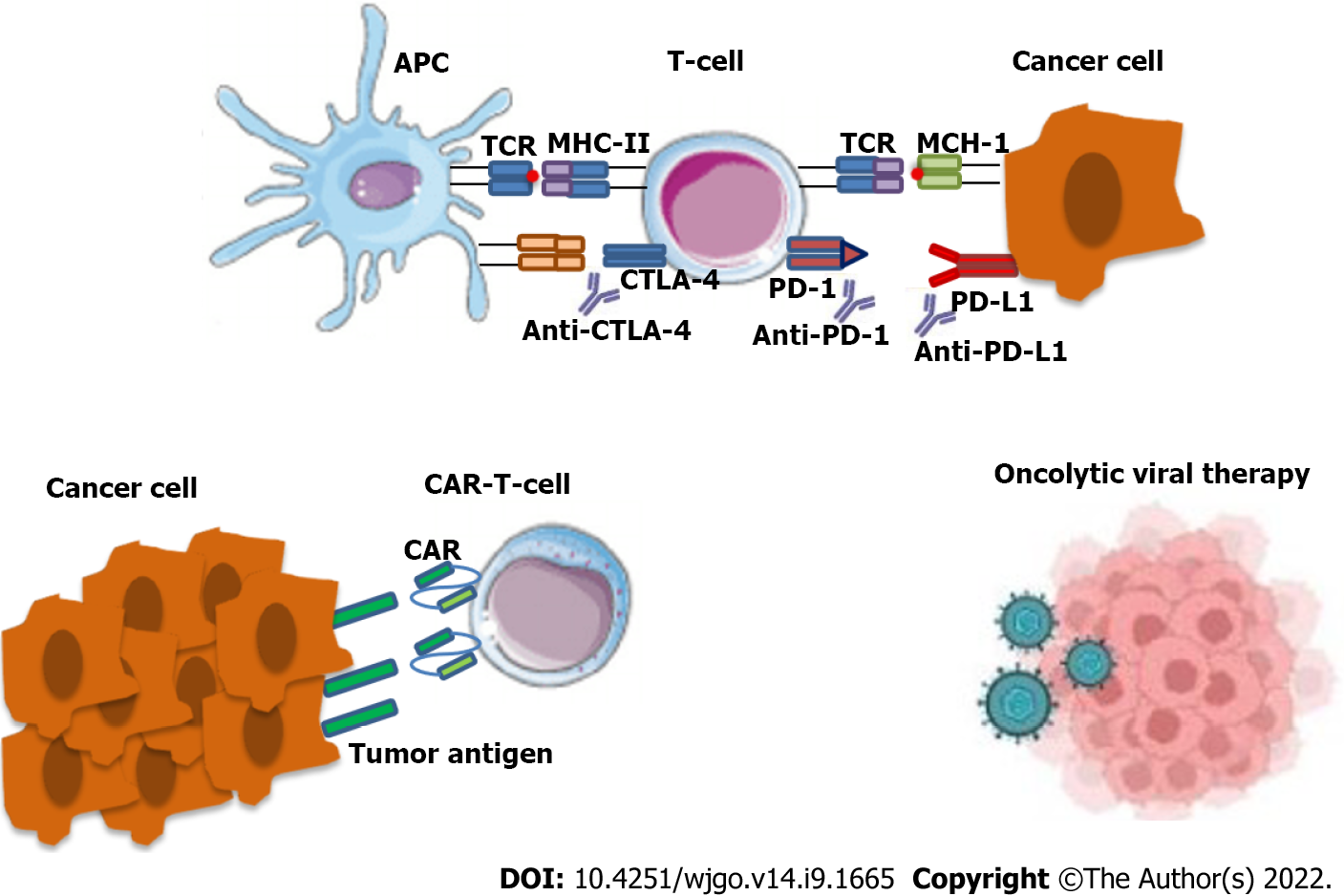
Figure 1 Mechanism of action of both anti anti-cytotoxic TT-lymphocyte antigen-4 and anti-programmed cell death protein 1/programmed cell death 1 ligand 1 check point inhibitors.
In the tumor microenvironment, antigen-presenting cells (APCs), such as dendritic cells processed specific tumor peptides (TAAs) and complexed them to major histocompatibility complex (MHC) molecules. Then, APC migrated to T cell-dependent areas of tumor presented TAA to naïve or quiescent T cells. Checkpoint inhibitor, such as anti-programmed cell death protein 1 (anti-PD-1)/anti-programmed cell death 1 ligand 1 (anti-PD-L1) and/oranti-cytotoxic TT-lymphocyte antigen-4 (anti-CTLA-4) on tumor cells, lead to re-activation of immune responses. The anti-PD-1 or anti-PD-L1 blocking by monoclonal antibodies (as nivolumab, pembrolizumab for PD-1 or atezolizumab for PD-L1) ipilimumab restore CD28 pro-activity signaling and restore effective anti-tumor T lymphocyte responses. The anti-CTLA-4 blocking by monoclonal antibodies as ipilimumab restore CD28 pro-activity signaling and result in effective anti-tumor T lymphocyte responses. The binding of PD-L1 to PD-1 and CTLA-4 to B7 keeps T cells from killing tumor cells in the body. Blocking the binding with an immune checkpoint inhibitor allows the T cells to kill tumor cells (upper panel).Chimeric antigen receptor (CAR) T cells are T cells that have been genetically engineered to produce an artificial T cell receptor for use in immunotherapy. CARs are receptor proteins that have been engineered to give T cells the new ability to target a specific protein (lower panel).
- Citation: Koustas E, Trifylli EM, Sarantis P, Papadopoulos N, Aloizos G, Tsagarakis A, Damaskos C, Garmpis N, Garmpi A, Papavassiliou AG, Karamouzis MV. Implication of gut microbiome in immunotherapy for colorectal cancer. World J Gastrointest Oncol 2022; 14(9): 1665-1674
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1665.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1665