Copyright
©The Author(s) 2022.
World J Gastrointest Oncol. Jul 15, 2022; 14(7): 1252-1264
Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1252
Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1252
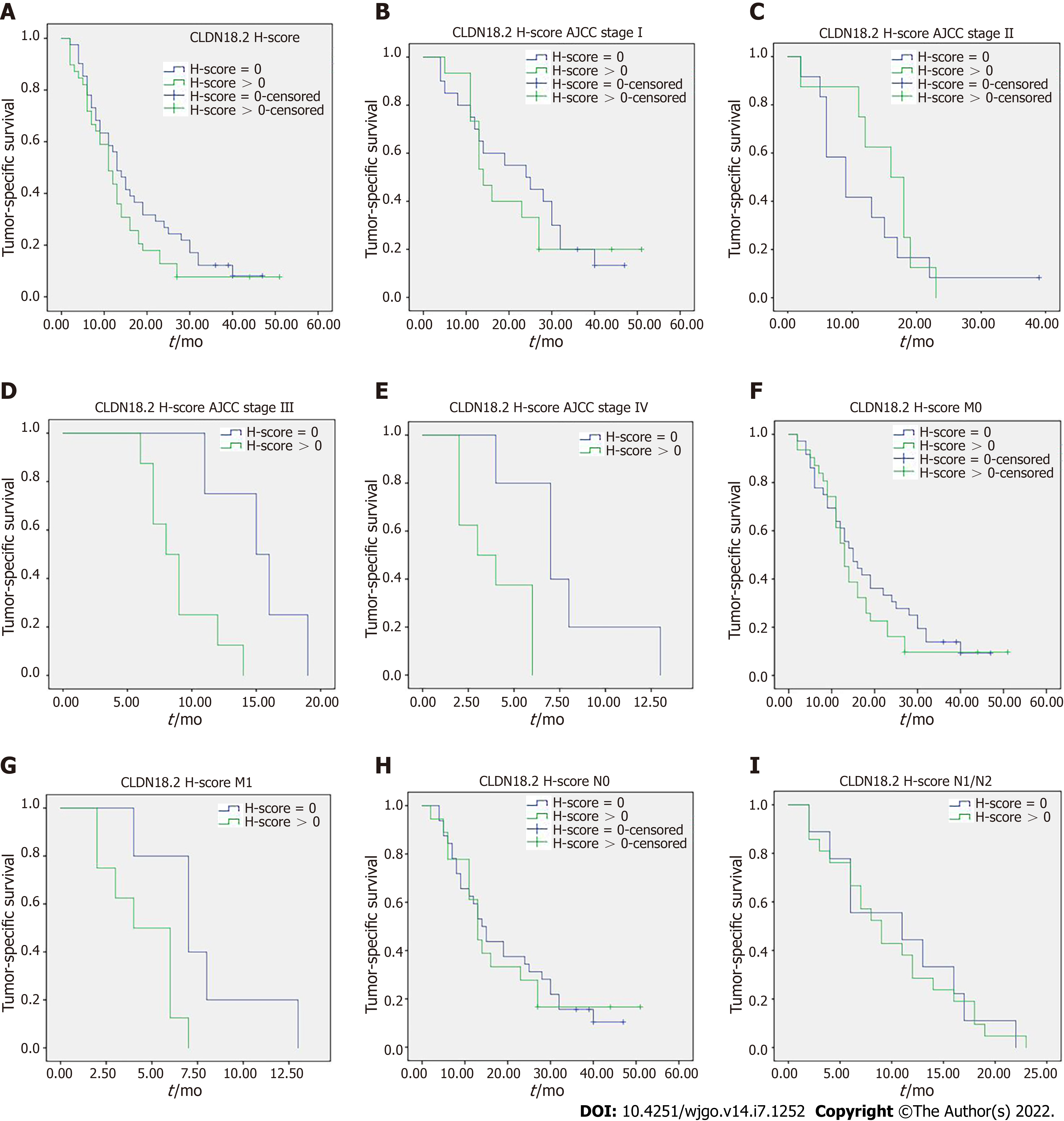
Figure 3 Claudin 18.
2 and survival. A: There was no significant correlation between tumor-specific survival and claudin 18.2 expression in tumor cells (41 vs 39 patients; median survival 13 mo vs 11 mo; P = 0.176); B: 20 vs 15 patients in stage I disease with median survival 24 mo vs 14 mo (P = 0.666); C: 12 vs 8 patients in stage II disease with median survival 9 mo vs 16 mo (P = 0.480); D: 4 vs 8 patients in stage III disease with median survival 15 mo vs 8 mo (P = 0.012); E: 5 vs 8 patients in stage IV disease with median survival 7 mo vs 3 mo (P = 0.009); F: 36 vs 31 patients in M0 disease with median survival 15 mo vs 13 mo (P = 0.351); G: 5 vs 8 patients in M1 disease with median survival 7 mo vs 4 mo (P = 0.024); H: 32 vs 18 patients in N0 disease with median survival 14 mo vs 13 mo (P = 0.825); I: 9 vs 21 patients in N1/N2 disease with median survival 11 mo vs 9 mo (P = 0.920). P values were obtained via log-rank-test. AJCC: American Joint Committee on Cancer; H-score: Histoscore.
- Citation: Wang X, Zhang CS, Dong XY, Hu Y, Duan BJ, Bai J, Wu YY, Fan L, Liao XH, Kang Y, Zhang P, Li MY, Xu J, Mao ZJ, Liu HT, Zhang XL, Tian LF, Li EX. Claudin 18.2 is a potential therapeutic target for zolbetuximab in pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2022; 14(7): 1252-1264
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1252.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1252