Copyright
©The Author(s) 2022.
World J Gastrointest Oncol. Jul 15, 2022; 14(7): 1252-1264
Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1252
Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1252
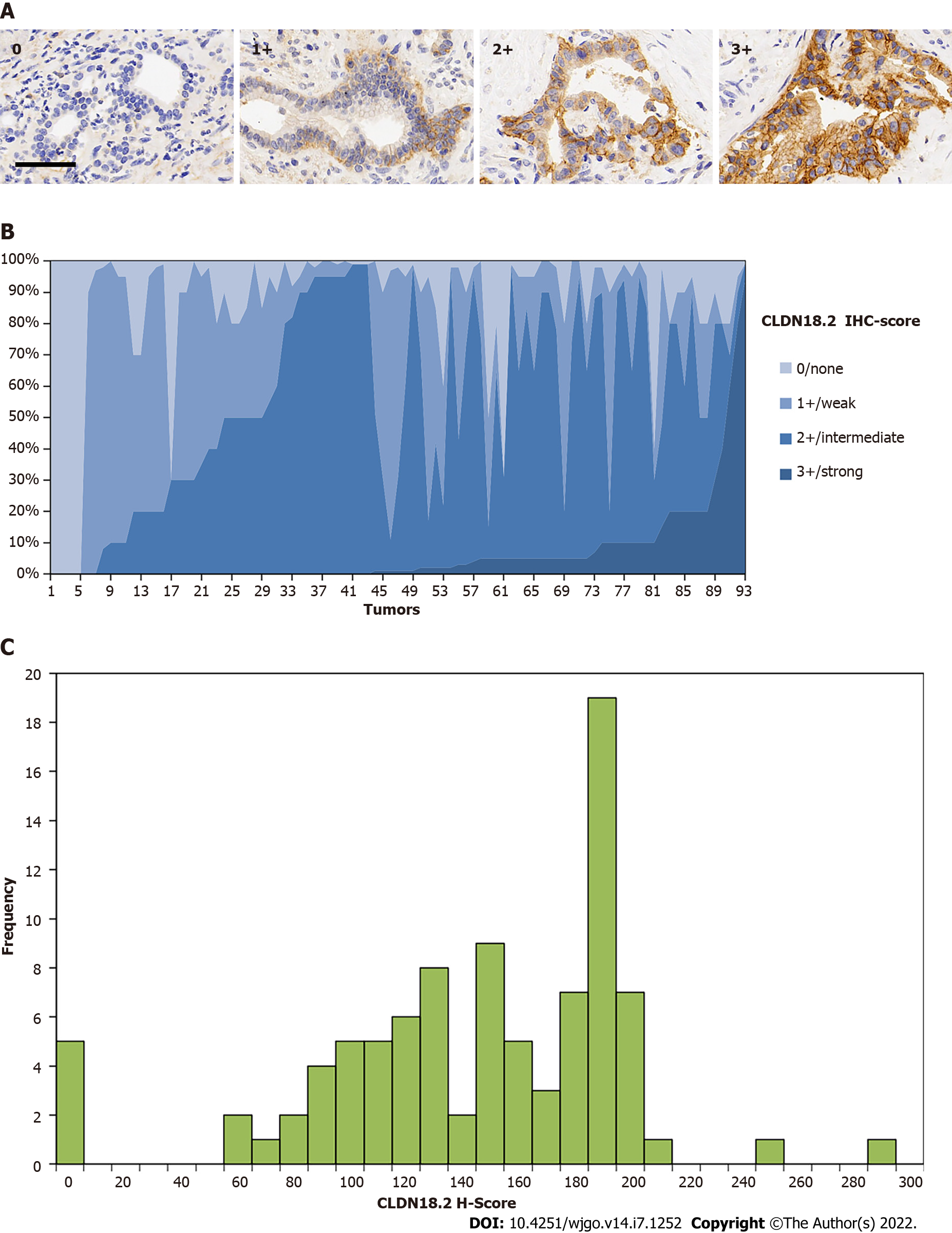
Figure 2 Expression of claudin 18.
2 in primary pancreatic ductal adenocarcinoma. A: Examples of claudin 18.2 (CLDN18.2)-positive pancreatic ductal adenocarcinoma tissues with 0/none, 1+/weak, 2+/intermediate, and 3+/ strong staining intensity. Scale bar 100 μm; B: Overall expression intensity of claudin 18.2. Eighty-eight (94.6%) primary pancreatic ductal adenocarcinoma tissues showed positivity for CLDN18.2 expression. In the positive cases, most showed compositive immunohistochemistry (IHC)-intensity. Fifty (56.8%) cases were scored up to IHC 3+, 86 (97.7%) cases were scored up to but no higher than IHC 2+, and 77 (87.5%) cases were no higher than IHC 1+; C: Histoscore (H-Score) distribution in the study. Minimum H-Score was 0; Maximum H-score was 292. Median H-score of positive tumors was 150.
- Citation: Wang X, Zhang CS, Dong XY, Hu Y, Duan BJ, Bai J, Wu YY, Fan L, Liao XH, Kang Y, Zhang P, Li MY, Xu J, Mao ZJ, Liu HT, Zhang XL, Tian LF, Li EX. Claudin 18.2 is a potential therapeutic target for zolbetuximab in pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2022; 14(7): 1252-1264
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1252.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1252