Published online Jun 16, 2023. doi: 10.4253/wjge.v15.i6.420
Peer-review started: April 3, 2023
First decision: May 12, 2023
Revised: May 15, 2023
Accepted: June 2, 2023
Article in press: June 2, 2023
Published online: June 16, 2023
Endoscopic vacuum therapy (EVT) is an increasingly popular treatment option for wall defects in the upper gastrointestinal tract. After its initial description for the treatment of anastomotic leaks after esophageal and gastric surgery, it was also implemented for a wide range of defects, including acute perforations, duodenal lesions, and postbariatric complications. Apart from the initially proposed hand
Core Tip: Endoscopic vacuum therapy (EVT) is a novel and effective endoscopic treatment option for anastomotic leaks and perforations in the upper gastrointestinal tract. Through the wide variety of available materials, EVT can be individually applied in almost every part of the oesophagus, the stomach and the duodenum with a clinical success rate of > 80% and low morbidity and mortality.
- Citation: Kouladouros K. Applications of endoscopic vacuum therapy in the upper gastrointestinal tract. World J Gastrointest Endosc 2023; 15(6): 420-433
- URL: https://www.wjgnet.com/1948-5190/full/v15/i6/420.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i6.420
The endoscopic treatment of wall defects in the upper gastrointestinal tract, both postoperative and acute/iatrogenic, is a challenging task for the endoscopist and requires a deep understanding of the pathophysiologic mechanisms involved as well as a high degree of expertise. The surgical approach, involving the closure of the defect and external drainage of the infected cavity, is technically challenging and associated with high morbidity and mortality, especially in difficult anatomic areas such as the intrathoracic esophagus and the duodenum[1,2]. The necessity of minimally invasive alternatives was therefore evident very early and various endoscopic methods have been implemented throughout the years, including endoscopic lavage, transmural drainage, and defect closure with clips, suturing, and stents[3]. Endoscopic vacuum therapy (EVT) was recently added to the spectrum of minimally invasive therapeutic options and quickly gained popularity, especially in Europe, mainly because of its tailored approach and its very good outcomes in a wide range of situations[4].
In this narrative review, we discuss the indications, technical aspects, and outcomes of EVT based on the current literature.
Vacuum therapy was initially introduced by plastic surgeons as a treatment option for chronic, infected, and ischemic wounds[5]. Its basic principle is that negative pressure applied to a secondary healing wound through a sealed system of a sponge applied onto the wound, with an airtight film covering it and a suction pump connected to it by a tube, accelerates the healing process through multiple mechanisms including: (1) Increased blood flow; (2) Local modulation of cytokines and chemoreceptor-modulated cell signaling, leading to enhanced neoangiogenesis and increased formation of granulation tissue; (3) Removal of debris and microorganisms; (4) Reduction of interstitial edema; (5) Continuous drainage of wound secretions; and (6) Macrodeformation of the wound with approximation of its edges and reduction of its volume[4-7].
After its initial application, the system needs to be changed regularly until adequate healing of the wound is achieved. Vacuum therapy quickly became an established treatment option for external wounds and in 2003 the first attempt was made to implement its principles for the treatment of an anastomotic leak in the rectum, practically treating the infected mesorectal cavity behind the anastomotic dehiscence as a chronic wound[8]. For that purpose, the sponge was mounted to a drain tube and then endoscopically inserted through the defect and into the cavity. Upon application of a vacuum to the other end of the tube, the cavity collapsed around the sponge, thus sealing the system without the need for a covering film. After the first successful implementation of EVT in the rectum, it started becoming popular for the treatment of rectal anastomotic leaks, and in 2008 it was used for the first time for similar defects in the upper gastrointestinal tract[9,10]. Initially, the method was only used in German centers. After the encouraging results of the first case series were published in subsequent years, EVT started to gain popularity and the first international reports from the United States and Korea were published in 2016, thus paving the way for its worldwide acceptance as a viable treatment option for defects of the gastrointestinal tract[11-14].
The main advantages of this novel approach in comparison to the already existing endoscopic treatment options, such as clips and stents, are its ability to facilitate the secondary healing of the defect without forcing an adaptation of the rigid, inflamed, and fibrotic edges and the possibility not only to cover the defect but also to properly drain the cavity behind it without the need for further external drainage. Disadvantages include the necessity of multiple endoscopic procedures, patient discomfort due to the transnasal tube, and reduced or prohibited oral intake because of the occlusion of the gastrointestinal tract, especially in the case of intraluminally placed devices.
EVT can be applied for all wall defects in the upper gastrointestinal tract as long as the following two conditions are fulfilled: There is adequate blood perfusion around the defect to allow for the tissue to react to the negative pressure, and there is a closed compartment that can collapse around the negative pressure device[15]. Although the size of the defect and the cavity plays an important role in the planning of the initial application and the duration of the therapy, large cavities and defects are not considered a contraindication. In one of the first published case series of Loske et al[11] in 2010 the size of the cavity was 3-40 mm, however recently published data have proven the feasibility of EVT in cavities > 7 cm and up to 15 cm, as long as they are closed and can collapse around the negative pressure device[11,16]. A large defect is associated with more intraprocedural difficulties and an increased number of procedures but also does not affect the outcomes of EVT[17].
The most common indications for EVT in the upper gastrointestinal tract are anastomotic leaks and acute perforations[18].
Anastomotic leaks after upper gastrointestinal tract surgery were the first indications for EVT described in the initial reports[9,10]. The incidence of anastomotic leaks after esophageal and gastric resections, typically presenting 7-10 d postoperatively, ranges between 5%-30% and is associated with increased morbidity and mortality rates of between 20%-50%[1,19,20]. In a Dutch cohort study including 1282 esophageal resections, an anastomotic leak was identified as the predominant specific complication associated with 30-d and 90-d mortality[21]. Surgical revision after esophagectomy has been associated with mortality of up to 64% and usually results in esophageal discontinuity[1,2]. Additionally, reversing esophageal discontinuity is not possible in 30% of the patients and even if it is attempted, it is associated with morbidity rates of up to 68%, long-term dysphagia in almost half of the patients, and sometimes the necessity of multiple surgical revisions[22-24]. Therefore, various conservative and minimally invasive regimes have been suggested, including thoracic drains and antibiotics, endoscopically placed transnasal drains, transmural drains (double-pigtail stents), defect adaptation with clips and suturing devices, and bridging of the leak with fully covered stents[25-29]. And yet, as Murphy pointed out in an editorial in 2010, the increasing number of treatment options for anastomotic dehiscence after esophagectomy reflects the difficulty in realizing a definitive therapy[3]. The introduction of EVT offered a powerful tool in the hands of the endoscopists, since it combines coverage of the defect and adequate drainage of the cavity behind the leak without the necessity of an additional, external drain and presented success rates of between 78%-100%[11,30-32]. Initially, EVT was used as a rescue therapy after failed surgical revision or stenting, but currently, it is being used as a first-line treatment for anastomotic leaks in many high-volume centers for upper gastrointestinal tract surgery[9,10,32]. Nevertheless, the endoscopist must bear in mind that although EVT can be applied for most anastomotic leaks, it is not suitable for very early leaks (within 4 d after surgery) with massive or complete anastomotic rupture and excessive tissue necrosis, and these patients should undergo surgical revision[30,32].
Acute perforations are nowadays usually iatrogenic and rarely spontaneous or traumatic. In contrast with anastomotic leaks, the edges of acute perforations are usually clean and lack inflammatory and fibrotic alterations, thus allowing for a better adaptation[33]. Additionally, no large cavity has been formed yet behind the defect. Therefore, closure of the perforation should be the primary therapeutic goal. Gomez-Esquivel suggested a therapeutic algorithm, according to which defects up to 2 cm should be primarily closed using though-the-scope clips. For defects between 2-3 cm over-the-scope-clips could be a better option and even larger defects should be covered by stents, as long as there is no cavity formed behind the defect. In the presence of a cavity, EVT should be considered the primary therapeutic option[34]. Early findings on the use of EVT for iatrogenic perforations and Boerhaave’s syndrome also showed a very high success rate of up to 100% with a minimum duration of therapy, but they are mostly based on small case series since in most centers EVT is considered a second-line treatment for acute perforations and thus is not as commonly implemented[35-37].
EVT has been reportedly used in all parts of the upper gastrointestinal tract and its feasibility and outcomes are primarily tied to technical aspects, including correct positioning of the device and the environment of the cavity.
The application of EVT for defects of the esophagus and intrathoracic anastomoses after esophageal surgery is the most commonly reported use[11,30]. The defects are usually easily reachable, and the associated cavities are restricted inside the mediastinum, thus facilitating the placement of the EVT device. Additionally, the high risk associated with esophageal emergency surgery and redo surgery further highlights the importance of EVT as a primary treatment in these cases[1]. Defects of the proximal esophagus have been described to be particularly difficult to treat, since negative pressure is more difficult to establish and maintain and the patient discomfort is maximized through the presence of a foreign body so close to the upper esophageal sphincter[4]. Nevertheless, several reports have demonstrated its feasibility, not only in the upper esophagus but also for pharyngeal defects after head and neck surgery[38,39].
Intraperitoneal gastric defects communicating with the abdominal cavity are typically not suitable for EVT and surgery should be preferred instead[4]. Additionally, intraluminal EVT is technically very difficult in the stomach, since its volume does not allow for the precise positioning of the EVT device in front of the defect and the complete collapse of the organ around it. Nevertheless, in the presence of a well-defined abscess cavity around an anastomotic leak or perforation it can be implemented, especially for patients showing poor tissue healing or who are unsuitable for surgery[40,41].
A special subgroup of gastric defects is those occurring after bariatric surgery. Staple line leaks occur in 1%-2% of patients after sleeve gastrectomy and 2%-5% of patients after Roux-en-Y gastric bypass[42,43]. Especially in the case of sleeve gastrectomy, leaks mostly occur at the proximal end of the staple line and are caused when the intragastric pressure exceeds the staple line resistance, whereas true ischemic leaks are rare[44,45]. Such leaks are characterized by a late onset of mild symptoms with up to 50% being asymptomatic, probably because of the amount of visceral fat restricting the leak and preventing generalized peritonitis[46,47]. Revision surgery after the second postoperative day has been proven to have insufficient results, thus shifting the focus toward endoscopic treatment options[46]. Several small series have reported the feasibility of EVT in these cases since 2016 and a recent analysis of 31 patients as well as a meta-analysis of 5 studies with a total of 55 patients showed high success rates of between 87%-90%[46,48].
Duodenal defects are technically more challenging and make the material selection more important. The long distance of the defect from the teeth is the main restricting factor when using an overtube, whereas the passage of the pyloric sphincter and the poor maneuverability of the endoscope inside the duodenum makes the positioning of a sponge in the piggyback technique more difficult. The slenderer variation of the open-pore film drainage (OFD), further explained below, can be placed more easily and seems to be an adequate alternative[49,50]. If technically feasible, EVT has been reported to have equally high success rates for duodenal defects, including perforations after endoscopic resections or endoscopic retrograde cholangiopancreatography and leaks after suture of perforated ulcers and intraoperative injuries, partially due to the additional benefit of removing the gastric and duodenal secretions from the area of the defect, which could potentially inhibit the healing process[50-53].
The use of EVT for rare indications, such as esophagobronchial fistulas and pancreatic necrosis has also been reported, but the evidence available is still insufficient[54,55].
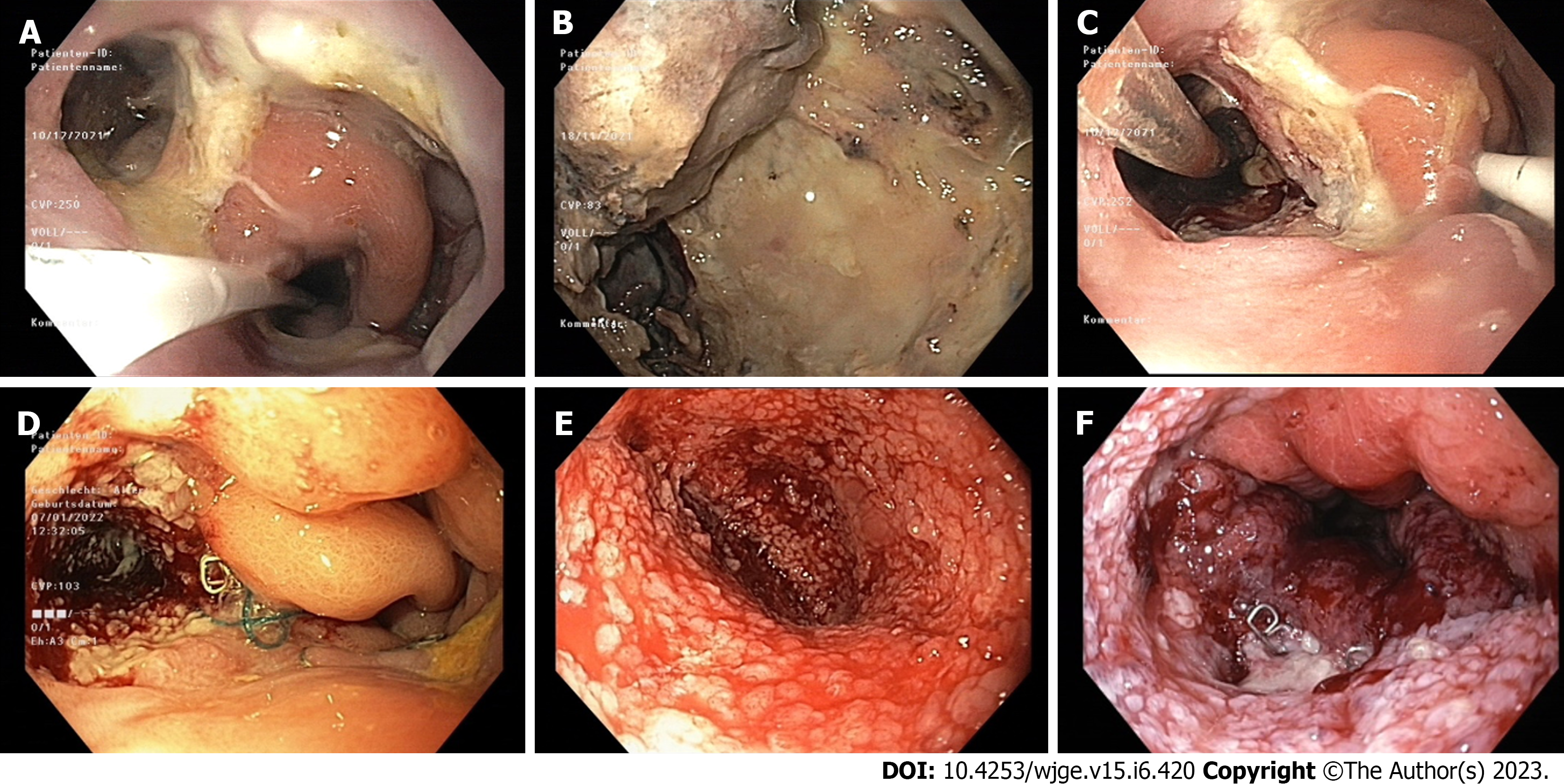
A combination of endoscopy and imaging techniques – most commonly computed tomography (CT) with oral contrast – is usually used to diagnose the defects and set the indication for EVT[4]. A careful endoscopic examination and documentation of the defect, the cavity behind it, and the blood perfusion around it are crucial for the planning of the initial procedure and the monitoring of the effects of EVT during the treatment[11]. CT scan provides additional information regarding the size and geometry of the cavity as well as its proximity to delicate anatomic structures, including the lung and large vessels.
The ideal placement of the EVT device has been thoroughly discussed in the literature. Traditional EVT consists of placing a sponge through the defect of the wall and inside the extraluminal cavity. The applied negative pressure causes the cavity to collapse, allows for sufficient drainage, and induces the formation of granular tissue on the walls of the cavity. During the subsequent EVT system changes the sponge is gradually retracted towards the lumen, thus leading to a downsizing of the cavity and finally to a closure of the defect (Figure 1). Alternatively, if the defect is too small, the EVT device can be placed intraluminally in front of it and the negative pressure is transferred to the cavity through the defect. Most experts suggest that, whenever technically possible, intracavitary EVT should be preferred, since it better reaches the entire cavity, thus enabling the healing from its most distal parts towards the lumen. At the same time, it prevents a superficial closure of the defect prior to the obliteration of the cavity with the formation of a closed, insufficiently drained space and ultimately an abscess[11,15,46,56-59]. Even in case of small wall defects, a prior balloon dilatation should be performed to enable the intracavitary placement of the EVT device, if a larger cavity is suspected[32]. A recent retrospective study with 119 patients also showed, that intraluminal placement of the EVT device is an independent risk factor for treatment failure, further supporting this strategy[60]. A further disadvantage of the intraluminal placement of the sponge is the complete occlusion of the lumen. As for the exact placement of the sponge inside the cavity, Loske et al[11] suggested that a small sponge at the entrance of the cavity is enough and can cause the entire cavity to collapse around it[11]. However, in this report, the maximum size of the cavities was 4 cm. In the case of larger cavities, this positioning could lead to a collapse of the proximal part of the cavity around the sponge with subsequent formation of granular tissue and gradual closure, thus separating the most distal parts and forming a second, insufficiently drained cavity. Therefore, we suggest the placement of a larger sponge up to the most distal part of the cavity during the initial procedure and a gradual withdrawal towards the lumen in the subsequent changes, in order to facilitate the gradual closure of the cavity from distal to proximal[16].
In case an intracavitary positioning is not possible and an intraluminal EVT has to be applied, a CT scan should be performed to exclude the presence of an insufficiently drained cavity, especially if the size of the defect does not allow for a thorough endoscopic exploration. A combination of both methods, simultaneously or in succession according to the changing geometry of the defect, is also possible.
The basic EVT system consists of the actual EVT device, usually a sponge or a drain, that is placed in the area where the negative pressure should be applied, an external negative pressure source, which may be a drain suction bottle or a pump, and tubing that connects these two elements and transfers the negative pressure from the external source to the EVT device. Regarding the negative pressure source, drain suction bottles are cheap and widely available, but the suction force they apply is unreliable and more difficult to control. Electronic pumps offer better control of the applied negative pressure; however, it has to be pointed out that most of the commercially available pumps are designed for and dedicated to external vacuum therapy and small adaptations are usually necessary to make them compatible with EVT devices[4].
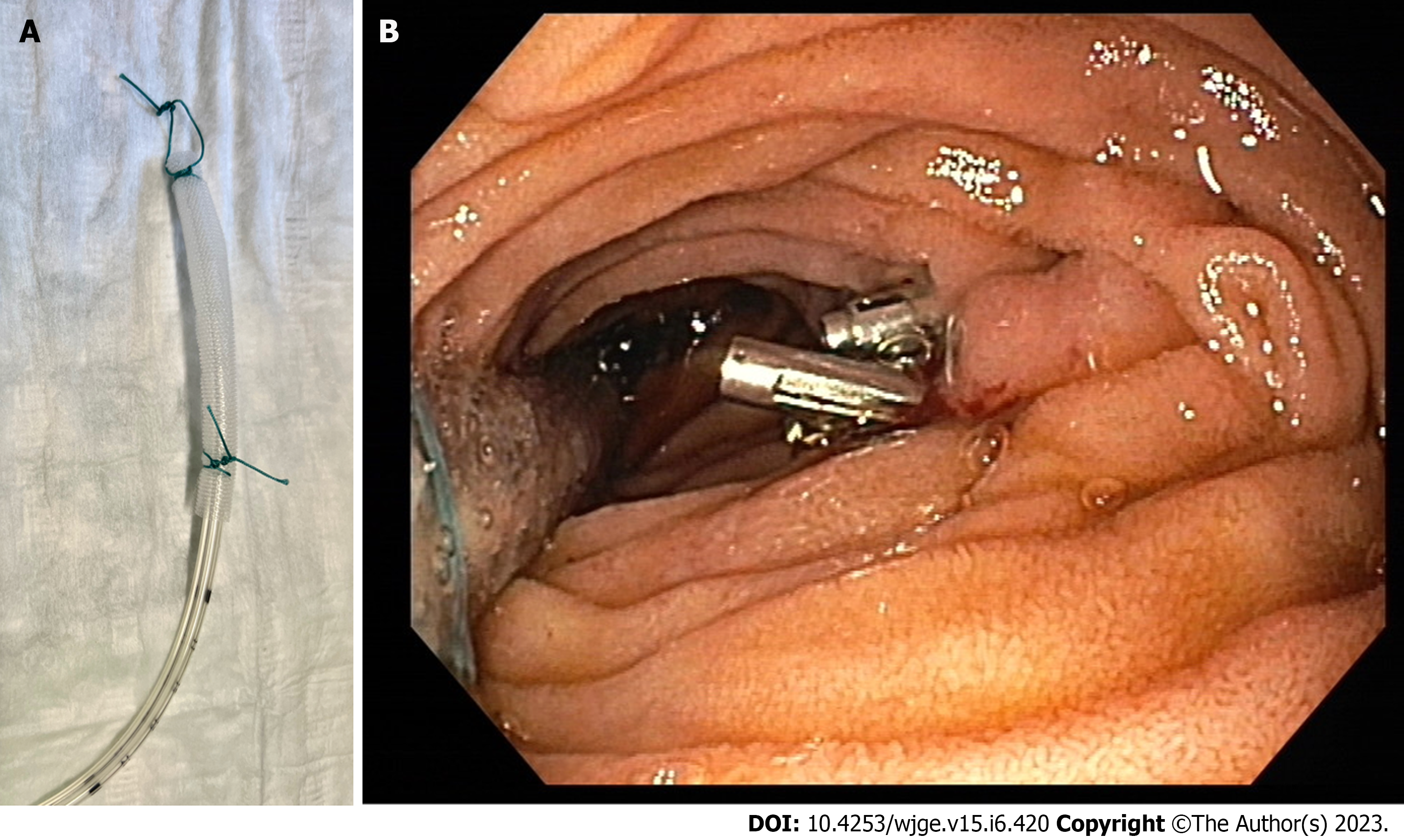
Regarding the EVT device, a large variety of alternatives has been suggested and the selection is usually based on the anatomic configuration of the defect, the availability of materials, and the experience of the endoscopist. In the initial description of endoscopic negative pressure therapy an individually prepared sponge was inserted using the “piggyback” technique[10]. According to this technique, a piece of polyurethane sponge designed for external negative pressure therapy is cut to the size of the cavity and attached around the perforated end of a surgical drain or a nasogastric tube (Figure 2). Macroporous, low-density sponges are preferred because of their greater debriding capacity and their stronger contraction under negative pressure, which leads to a more pronounced shrinkage of the cavity, although this structure allows for more tissue ingrowth thus making removal more difficult[4]. The distal end of the drain is shortened accordingly so that no perforations lie outside of the sponge. An endoscopic forceps is inserted into the instrument channel of the endoscope and the tip of the sponge or a loop attached to its distal end is grasped. The sponge is then inserted parallel to the endoscope and placed, with the use of the forceps, in its proper position. This method is cheap and versatile, allowing for the individual construction of the sponge according to the needs of every patient. However, the “piggyback” technique is technically demanding and the parallel insertion of the sponge and the endoscope restricts the field of view and might increase the risk of injury, especially in organs with a narrow lumen, like the esophagus. This method was predominantly used in earlier studies and is still being used in many centers with high success rates[32,57,58,60].
The EsoSponge System (B. Braun Medical Ltd, Sheffield, United Kingdom) became available in 2014 as a variation of the EndoSponge System designed for rectal EVT and is still the only commercially available EVT sponge. It consists of a 55 mm long, 15 mm wide macroporous sponge fixed at the end of a 100 cm drainage tube, an overtube, and a pusher (Figure 2). The endoscope is inserted into the overtube and then inserted through the defect into the cavity. The overtube is slid over the endoscope until its tip is inside the cavity and then the endoscope is removed, leaving the overtube in place. The sponge is inserted into the overtube and pushed through it with the help of the pusher. When the entire pusher is inside the overtube, the sponge has been completely released in front of the tip of the overtube and inside the cavity. In the case of intraluminal EVT the tip of the overtube is placed inside the lumen, directly proximal to the defect, and the sponge is released in the lumen, at the entrance of the cavity. The position of the sponge is controlled endoscopically and corrected if necessary. This procedure is standardized and technically easier to perform, while the overtube protects the esophageal wall from potential injuries and separates the gastrointestinal tract from the airway, thus reducing the risk of aspiration. The sponge can also be modified according to the geometry of each cavity and even extended with the attachment of additional pieces of sponge if necessary[16]. Large retrospective studies have shown high success rates with the use of the EsoSponge System, however, none compare it to the “piggyback” technique, so the final decision lies at the discretion of the endoscopist[11,30,52,61,62].
In 2015 Loske et al[49] described the OFD tool, an alternative EVT device consisting of a nasogastric tube with its distal, perforated end wrapped in a very thin, double-layered, open-pore drainage film (Figure 3). The drainage film is fixed around the tube with a suture, the tube is inserted through the nose like a normal nasogastric tube and the distal tip is positioned through the defect or in front of it with the use of an endoscopic forceps[49]. This device is much smaller than the sponges, can be easily placed through smaller defects or in difficult positions, like the duodenum, and can be left in place longer, since it is not as prone to tissue ingrowth as other devices. The first reports on OFD have shown encouraging results for various indications, including duodenal lesions and preemptive EVT after esophageal resection[63-65].
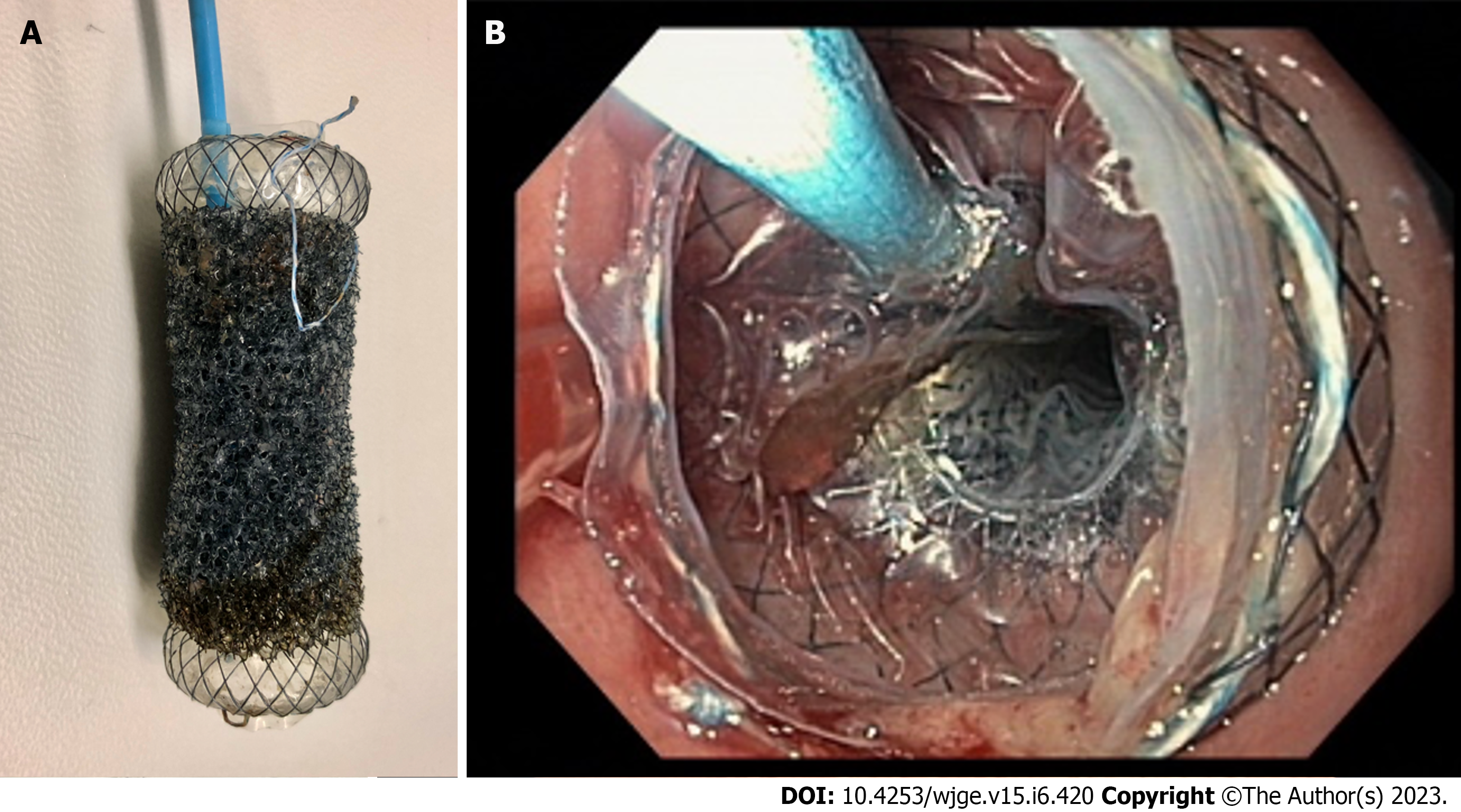
A further development was the introduction of the VAC-Stent (MICRO-TECH Europe GmbH, Düsseldorf, Germany), a fully covered, 70 mm long and 14 mm wide self-expanding metal stent (SEMS) with its central 50 mm part covered by a macroporous polyurethane sponge connected to a tube that can be connected to a negative pressure source (Figure 4). This product combines the advantages of intraluminal EVT with a lack of occlusion of the gastrointestinal lumen, thus allowing oral intake and reducing patient discomfort. Since it can only be placed intraluminally, it is suitable for defects without large associated cavities, although it can also be placed over an intracavitary sponge, combining both methods. The first published series showed a success rate of 80%, although a prominent selection bias of these studies has to be taken into consideration[66,67].
The initial placement of the EVT device and the subsequent procedures can be performed in the endoscopy suite under sedation, provided that the patient’s condition supports that. The initial procedure takes usually slightly longer than the following ones and the average procedure time is between 30-60 min[68,69]. The partial or complete occlusion of the lumen is an important issue and the placement of a feeding tube distal to the EVT device is generally advised. Especially in the case of Ivor-Lewis esophagectomy with delayed gastric emptying the placement of a dual-lumen tube should be considered, with the longer feeding tube positioned postpyloric and the proximal gastric tube in the stomach to facilitate the evacuation of gastric secretions[30].
There is no consensus regarding the settings of the negative pressure, mainly because of the complete lack of evidence on this subject. Initially the pressure of -125 mmHg usually used in external negative pressure therapy was also used for EVT and pressure settings between -100 and -125 mmHg have been used in the largest published series to date[30,32,60]. Other authors suggest lower pressure settings of -20 to -50 mmHg in order to prevent bleeding, injuries, and formation of fistulas, especially when the EVT device lies in close proximity to delicate structures[70]. Still, the standard pressure settings vary greatly from center to center and this was also depicted in an international survey published in 2019[59].
A further issue subject to a lot of debate is the ideal interval between changes of the EVT system. It is known from external negative pressure therapy that the sponge becomes occluded by tissue ingrowth and wound secretions and has to be changed regularly. Most of the experts suggest an interval of 3-5 d[15,59,71]. However, the lack of further evidence has led to a wide spread of different strategies used in different centers and this is also depicted in the literature, with some authors supporting a shorter interval of 2-3 d so as to prevent excessive tissue ingrowth and others opting for an interval of 7 d, aiming to reduce the number of procedures needed[60,61,72,73]. Intervals over 7 d are generally discouraged, since the sponge might be embedded in the tissue thus making its removal difficult and increasing the risk of injuries, but apart from that the decision is usually made according to center standards and individual patient characteristics[7].
The negative pressure has to be relieved prior to any subsequent endoscopic procedure in order to facilitate the removal of the sponge. If the device is firmly attached to the tissue, it may be rinsed with water and then carefully dislodged from the surrounding tissue with the tip of the endoscope before being pulled back[58]. The defect and the cavity should be carefully reevaluated every time so that the therapy can be adapted accordingly. It has also been shown that the actual size of the defect or the cavity might not be evident during initial endoscopy, being masked behind debris or necrotic tissue, and only revealed in the subsequent procedures[16].
The main criterion for termination of EVT is the formation of a shallow cavity with a wide entrance and adequate drainage into the lumen, covered by healthy granular tissue[13,16]. The exact maximum cavity size required to end EVT varies between 0.5 and 3 cm from study to study, but geometry also plays an important role[74]. Additional treatment of residual defects and fistulas with the use of clips or fibrin glue has also been reported[32,75]. On the other hand, if no signs of tissue reaction and progress, both endoscopic and clinical, are evident after 3 wk of EVT, an alternative treatment should be considered[32].
Published studies report success rates of EVT in the upper gastrointestinal tract ranging between 78%-100%, although most of them are retrospective and based on very heterogenous populations[30-32,46,62,76]. These findings have been verified in 3 meta-analyses with pooled success rates between 81%-87%[48,77,78]. When applied in the esophagus, clinical success seems to be higher for more distal defects and the results are better when applied as a first-line treatment in comparison to rescue treatment after failed stenting or surgical revision[62,77]. The type of defect does not seem to affect the success rate, although acute perforations tend to need fewer procedures and a shorter overall duration of treatment[30,35].
The experience of the endoscopist is crucial for the technical and clinical success of EVT. A recent study evaluated outcomes in one clinic over a period of 10 years and noticed an increase in success rates from 80% to 91%, while therapy duration and the need for additional treatments and redo surgery significantly decreased with accumulating experience[17]. Ward et al[69] argued that technical proficiency can be achieved after the first 10 procedures, but this is largely dependent on the severity of the case and the geometry and localization of the defect[69].
The duration of treatment and the number of necessary procedures vary greatly and depend on the type of defect, the size of the defect and the associated cavity, and the healing potential of the patient. Most studies report 3-6 subsequent endoscopic procedures in a period of 11-25 d, with a tendency towards a shorter duration of treatment in cases of acute perforations and defects in the duodenum[14,30,32,35,52,76].
The rate of immediate adverse events is generally low and reaches 10% for the entire course of treatment[77]. These mostly include dislocation of the EVT device, mild bleeding after removal, and aspiration pneumonia[60]. Some rare but potentially life-threatening complications have been reported when the sponge came into close proximity to large vessels and eroded them, leading to uncontrolled and sometimes fatal bleeding, but to our knowledge, only 5 such cases have been reported so far[32,61,73]. Nevertheless, the severity of these complications points out the importance of careful evaluation of the cavity and, when in doubt, of an additional CT scan to assess the relative position of the sponge to delicate structures[32]. Bronchoesophageal fistulas have also been rarely reported, although in these cases it is difficult to differentiate if they were caused by EVT or the initial leak itself[57,79]. On the other hand, the most common long-term complications are strictures in the area affected by the EVT. The stricture rate lies between 8%-20%, but almost all of them can be successfully treated by endoscopic dilatation[60,61,77].
A wide range of alternative treatments have been described for the treatment of upper gastrointestinal defects. Clips and suturing methods show high success rates in cases of acute perforations with small defects and no associated cavity[33,80]. Transluminal drainage with the use of transnasal tubes or pigtails has also been reported, but the evidence is still low and this method is mostly used in selected patients[27,29,81]. Stents are the most common treatment alternative to EVT. Until the introduction of EVT stenting was the primary treatment option for large defects and especially anastomotic leaks in the upper gastrointestinal tract, with clinical success rates between 80%-90%[28,82-84]. SEMS were used in most of the published studies and complications include stent migration, strictures, and aortoesophageal fistulas with potentially fatal bleeding[83,85,86]. The main disadvantage of SEMS, though, is the lack of drainage of the cavity behind the defect. Several retrospective studies and two metanalyses have compared treatment outcomes between EVT and SEMS, generally showing higher success rates, reduced duration of therapy, and lower rates of adverse events for EVT[57,75,87-90]. Only one study found no difference between the two treatment options in any of the parameters mentioned above; the cross-over between the two groups was however significant and might have influenced the results[91]. The protocol of the ESOLEAK study, a phase 2 randomized trial comparing EVT and SEMS in the upper gastrointestinal tract, was published in 2021. The study is currently recruiting and to our knowledge, no results have been published so far[92].
Based on the fact that EVT facilitates healing, several efforts have been made to implement it prophylactically on high-risk anastomoses in order to reduce the incidence of anastomotic leaks. The main principle of preemptive EVT is that it can treat small, undetectable defects of the anastomosis and prevent the contamination of the mediastinum, thus leading to their closure before they become clinically evident[93]. In 2017 Neumann et al[94] suggested a scheduled endoscopic control of the anastomosis several days after esophagectomy with the application of preemptive EVT if the tissue showed any signs of ischemia. In this first series of 8 patients, the anastomotic leak rate was still 25% and 3 patients developed strictures[94]. Four further studies evaluated the intraoperative placement of a sponge or an OFD intraluminally at the area of the anastomosis and reevaluated 3-6 d later. In the case of high-risk findings during control-endoscopy, including visible suture material, fibrin, and ischemia, EVT was prolonged, otherwise it was terminated. The reported anastomotic leak rates varied between 0%-7.5%, which is lower than usually reported, but the series was small and there was no control group to verify its positive effects[65,93,95,96]. Therefore, preemptive EVT still remains an attractive theory, but further data is required to prove its efficacy and determine the patient groups that could profit from it.
In summary, we can conclude that EVT is an adequate treatment option for wall defects in the upper gastrointestinal tract, with high success rates and low morbidity. The available evidence has proved its efficacy in different localizations and clinical settings for both acute perforations and anastomotic leaks and especially for the latter it is considered a first-line treatment in many centers. However, the data regarding the technical aspects, including choice of materials, pressure settings, and procedure interval, are scarce, and further randomized trials are necessary to clarify those points.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sano W, Japan; Spadaccini M, Italy S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Lang H, Piso P, Stukenborg C, Raab R, Jähne J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol. 2000;26:168-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 176] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Orringer MB. Reversing esophageal discontinuity. Semin Thorac Cardiovasc Surg. 2007;19:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Murphy T, Jobe BA. Endoluminal management of anastomotic dehiscence after esophagectomy: an increasing quiver of options reflects the difficulty in realizing a definitive therapy. Gastrointest Endosc. 2010;71:387-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Gutschow CA, Schlag C, Vetter D. Endoscopic vacuum therapy in the upper gastrointestinal tract: when and how to use it. Langenbecks Arch Surg. 2022;407:957-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563-76; discussion 577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1531] [Cited by in F6Publishing: 1325] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 6. | Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg. 2014;101:1627-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | de Moura DTH, de Moura BFBH, Manfredi MA, Hathorn KE, Bazarbashi AN, Ribeiro IB, de Moura EGH, Thompson CC. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc. 2019;11:329-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 78] [Cited by in F6Publishing: 73] [Article Influence: 14.6] [Reference Citation Analysis (6)] |
| 8. | Weidenhagen R, Spelsberg F, Lang RA, Jauch KW, Gruetzner KU. A New Method for Sepsis Control Caused by Anastomotic Leakage in Rectal Surgery - the Endo-VAC. Colorectal Disease. 2003;5:1-4. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Wedemeyer J, Schneider A, Manns MP, Jackobs S. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc. 2008;67:708-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Loske G, Müller C. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc. 2009;69:601-2; author reply 602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Loske G, Schorsch T, Müller C. Endoscopic vacuum sponge therapy for esophageal defects. Surg Endosc. 2010;24:2531-2535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12:1278-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Smallwood NR, Fleshman JW, Leeds SG, Burdick JS. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc. 2016;30:2473-2480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Hwang JJ, Jeong YS, Park YS, Yoon H, Shin CM, Kim N, Lee DH. Comparison of Endoscopic Vacuum Therapy and Endoscopic Stent Implantation With Self-Expandable Metal Stent in Treating Postsurgical Gastroesophageal Leakage. Medicine (Baltimore). 2016;95:e3416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Loske G, Müller CT. Tips and tricks for endoscopic negative pressure therapy. Chirurg. 2019;90:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Kouladouros K, Belle S, Reissfelder C, Kähler G. Endoscopic negative pressure therapy for leaks with large cavities in the upper gastrointestinal tract: is it a feasible therapeutic option? Scand J Gastroenterol. 2021;56:193-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Reimer S, Lock JF, Flemming S, Weich A, Widder A, Plaßmeier L, Döring A, Hering I, Hankir MK, Meining A, Germer CT, Groneberg K, Seyfried F. Endoscopic Management of Large Leakages After Upper Gastrointestinal Surgery. Front Surg. 2022;9:885244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 18. | Schorsch T, Müller C, Loske G. Endoscopic vacuum therapy of anastomotic leakage and iatrogenic perforation in the esophagus. Surg Endosc. 2013;27:2040-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg. 2004;10:71-75. [PubMed] [Cited in This Article: ] |
| 20. | Ikeguchi M, Oka S, Gomyo Y, Tsujitani S, Maeta M, Kaibara N. Postoperative morbidity and mortality after gastrectomy for gastric carcinoma. Hepatogastroenterology. 2001;48:1517-1520. [PubMed] [Cited in This Article: ] |
| 21. | Talsma AK, Lingsma HF, Steyerberg EW, Wijnhoven BP, Van Lanschot JJ. The 30-day versus in-hospital and 90-day mortality after esophagectomy as indicators for quality of care. Ann Surg. 2014;260:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Barkley C, Orringer MB, Iannettoni MD, Yee J. Challenges in reversing esophageal discontinuity operations. Ann Thorac Surg. 2003;76:989-94; discussion 995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Braghetto I, Cardemil G, Csendes A, Venturelli A, Herrera M, Korn O, Sepúlveda S, Rojas J. Digestive tract reconstitution after failed esophago-gastro or esophago-coloanastomosis. Arq Bras Cir Dig. 2013;26:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Parekh K, Iannettoni MD. Complications of esophageal resection and reconstruction. Semin Thorac Cardiovasc Surg. 2007;19:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Matory YL, Burt M. Esophagogastrectomy: reoperation for complications. J Surg Oncol. 1993;54:29-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Nguyen NT, Hinojosa MW, Fayad C, Wilson SE. Minimally invasive management of intrathoracic leaks after esophagogastrectomy. Surg Innov. 2007;14:96-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Hu Z, Yin R, Fan X, Zhang Q, Feng C, Yuan F, Chen J, Jiang F, Li N, Xu L. Treatment of intrathoracic anastomotic leak by nose fistula tube drainage after esophagectomy for cancer. Dis Esophagus. 2011;24:100-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Amrani L, Ménard C, Berdah S, Emungania O, Soune PA, Subtil C, Brunet C, Grimaud JC, Barthet M. From iatrogenic digestive perforation to complete anastomotic disunion: endoscopic stenting as a new concept of "stent-guided regeneration and re-epithelialization". Gastrointest Endosc. 2009;69:1282-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Jung CFM, Hallit R, Müller-Dornieden A, Calmels M, Goere D, Chaput U, Camus M, Gonzalez JM, Barthet M, Jacques J, Legros R, Barrioz T, Kück F, Seif Amir Hosseini A, Ghadimi M, Kunsch S, Ellenrieder V, Wedi E, Barret M. Endoscopic internal drainage and low negative-pressure endoscopic vacuum therapy for anastomotic leaks after oncologic upper gastrointestinal surgery. Endoscopy. 2022;54:71-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Bludau M, Fuchs HF, Herbold T, Maus MKH, Alakus H, Popp F, Leers JM, Bruns CJ, Hölscher AH, Schröder W, Chon SH. Results of endoscopic vacuum-assisted closure device for treatment of upper GI leaks. Surg Endosc. 2018;32:1906-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Kuehn F, Loske G, Schiffmann L, Gock M, Klar E. Endoscopic vacuum therapy for various defects of the upper gastrointestinal tract. Surg Endosc. 2017;31:3449-3458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D, Senninger N, Vowinkel T. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc. 2017;31:2687-2696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 118] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 33. | Mennigen R, Senninger N, Laukoetter MG. Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the-scope clips. World J Gastroenterol. 2014;20:7767-7776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 89] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Gomez-Esquivel R, Raju GS. Endoscopic closure of acute esophageal perforations. Curr Gastroenterol Rep. 2013;15:321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Loske G, Schorsch T, Dahm C, Martens E, Müller C. Iatrogenic perforation of esophagus successfully treated with Endoscopic Vacuum Therapy (EVT). Endosc Int Open. 2015;3:E547-E551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Loske G, Schorsch T, van Ackeren V, Schulze W, Müller CT. Endoscopic vacuum therapy in Boerhaave's syndrome with open-pore polyurethane foam and a new open-pore film drainage. Endoscopy. 2015;47 Suppl 1 UCTN:E410-E411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Loske G, Schorsch T. [Endoscopic vacuum therapy for Boerhaave's syndrome]. Chirurg. 2016;87:676-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Lenzen H, Negm AA, Erichsen TJ, Manns MP, Wedemeyer J, Lankisch TO. Successful treatment of cervical esophageal leakage by endoscopic-vacuum assisted closure therapy. World J Gastrointest Endosc. 2013;5:340-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Loeck J, von Lücken HJ, Münscher A, Müller CT, Loske G. Endoscopic negative pressure therapy (ENPT) in head and neck surgery: first experiences in treatment of postoperative salivary fistulas and cervical esophageal perforations. Eur Arch Otorhinolaryngol. 2021;278:4525-4534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Loske G, Lang U, Schorsch T, Müller CT. [Complex vacuum therapy of an abdominal abscess from gastric perforation : case report of innovative operative endoscopic management]. Chirurg. 2015;86:486-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Senne M, Werner CR, Schempf U, Thiel K, Königsrainer A, Wichmann D. Comparison of Two Endoscopic Therapeutic Interventions as Primary Treatment for Anastomotic Leakages after Total Gastrectomy. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 42. | Noel P, Nedelcu M, Gagner M. Impact of the Surgical Experience on Leak Rate After Laparoscopic Sleeve Gastrectomy. Obes Surg. 2016;26:1782-1787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | ASMBS Clinical Issues Committee. ASMBS guideline on the prevention and detection of gastrointestinal leak after gastric bypass including the role of imaging and surgical exploration. Surg Obes Relat Dis. 2009;5:293-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M. The science of stapling and leaks. Obes Surg. 2004;14:1290-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 45. | Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014;10:713-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 46. | Markus A, Henrik BJ, Benedikt R, Alexander H, Thomas B, Clemens S, Jan-Hendrik E. Endoscopic vacuum therapy in salvage and standalone treatment of gastric leaks after bariatric surgery. Langenbecks Arch Surg. 2022;407:1039-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Bhayani NH, Swanström LL. Endoscopic therapies for leaks and fistulas after bariatric surgery. Surg Innov. 2014;21:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Intriago JMV, de Moura DTH, do Monte Junior ES, Proença IM, Ribeiro IB, Sánchez-Luna SA, Bernardo WM, de Moura EGH. Endoscopic Vacuum Therapy (EVT) for the Treatment of Post-Bariatric Surgery Leaks and Fistulas: a Systematic Review and Meta-analysis. Obes Surg. 2022;32:3435-3451. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 49. | Loske G, Rucktäschel F, Schorsch T, van Ackeren V, Stark B, Müller CT. Successful endoscopic vacuum therapy with new open-pore film drainage in a case of iatrogenic duodenal perforation during ERCP. Endoscopy. 2015;47:E577-E578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Loske G, Rucktaeschel F, Schorsch T, Moenkemueller K, Mueller CT. Endoscopic negative pressure therapy (ENPT) for duodenal leakage - novel repair technique using open-pore film (OFD) and polyurethane-foam drainages (OPD). Endosc Int Open. 2019;7:E1424-E1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Abbitt D, Barnes AL, Hammad HT, Reveille RM, Jones EL. Endoluminal vacuum closure of a duodenal perforation. J Surg Case Rep. 2021;2021:rjab479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 52. | Chevallay M, Lorenz F, Bichard P, Frossard JL, Schmidt T, Goeser T, Bruns CJ, Mönig SP, Chon SH. Outcome of endoscopic vacuum therapy for duodenal perforation. Surg Endosc. 2023;37:1846-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 53. | Wichmann D, Jansen KT, Onken F, Stüker D, Zerabruck E, Werner CR, Yurttas C, Thiel K, Königsrainer A, Quante M. Endoscopic negative pressure therapy as stand-alone treatment for perforated duodenal diverticulum: presentation of two cases. BMC Gastroenterol. 2021;21:436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Loske G, Schorsch T, Gobrecht O, Martens E, Rucktäschel F. Transgastric endoscopic vacuum therapy with a new open-pore film drainage device in a case of infective pancreatic necrosis. Endoscopy. 2016;48 Suppl 1:E148-E149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Kuckelman J, Bryan D, Wiener D. Endoluminal Vacuum Therapy for Definitive Management of an Esophagobronchial Fistula. Ann Thorac Surg. 2022;113:669-673. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 56. | Loske G, Schorsch T, Müller C. Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy. 2011;43:540-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer J, Manns MP, Schneider AS, Wedemeyer J. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy. 2013;45:433-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 58. | Leeds SG, Mencio M, Ontiveros E, Ward MA. Endoluminal Vacuum Therapy: How I Do It. J Gastrointest Surg. 2019;23:1037-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 59. | Rodrigues-Pinto E, Repici A, Donatelli G, Macedo G, Devière J, van Hooft JE, Campos JM, Galvao Neto M, Silva M, Eisendrath P, Kumbhari V, Khashab MA. International multicenter expert survey on endoscopic treatment of upper gastrointestinal anastomotic leaks. Endosc Int Open. 2019;7:E1671-E1682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Jung DH, Huh CW, Min YW, Park JC. Endoscopic vacuum therapy for the management of upper GI leaks and perforations: a multicenter retrospective study of factors associated with treatment failure (with video). Gastrointest Endosc. 2022;95:281-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 61. | Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens A, Broering DC, Schniewind B. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy. 2010;42:693-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Richter F, Hendricks A, Schniewind B, Hampe J, Heits N, von Schönfels W, Reichert B, Eberle K, Ellrichmann M, Baumann P, Egberts JH, Becker T, Schafmayer C. Eso-Sponge® for anastomotic leakage after oesophageal resection or perforation: outcomes from a national, prospective multicentre registry. BJS Open. 2022;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Loske G, Schorsch T, Rucktaeschel F, Schulze W, Riefel B, van Ackeren V, Mueller CT. Open-pore film drainage (OFD): a new multipurpose tool for endoscopic negative pressure therapy (ENPT). Endosc Int Open. 2018;6:E865-E871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 64. | Wichmann D, Stüker D, Schempf U, Werner CR, Steger V, Königsrainer A, Schweizer U, Archid R. Endoscopic negative pressure therapy with open-pore film drainage and open-pore polyurethane sponge drainage for iatrogenic perforation of the esophagus. Endoscopy. 2020;52:377-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Loske G, Müller J, Schulze W, Riefel B, Müller CT. Pre-emptive active drainage of reflux (PARD) in Ivor-Lewis oesophagectomy with negative pressure and simultaneous enteral nutrition using a double-lumen open-pore film drain (dOFD). Surg Endosc. 2022;36:2208-2216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 66. | Lange J, Kähler G, Bernhardt J, Knievel J, Dormann A, Hügle U, Eisenberger CF, Heiss MM. The VACStent trial: combined treatment of esophageal leaks by covered stent and endoscopic vacuum therapy. Surg Endosc. 2023;37:3657-3668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 67. | Chon SH, Töx U, Lorenz F, Rieck I, Wagner BJ, Kleinert R, Fuchs HF, Goeser T, Quaas A, Bruns CJ. A Novel Hybrid Stent with Endoscopic Vacuum Therapy for Treating Leaks of the Upper Gastrointestinal Tract. Visc Med. 2021;37:403-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Ooi G, Burton P, Packiyanathan A, Loh D, Chen R, Shaw K, Brown W, Nottle P. Indications and efficacy of endoscopic vacuum-assisted closure therapy for upper gastrointestinal perforations. ANZ J Surg. 2018;88:E257-E263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Ward MA, Hassan T, Burdick JS, Leeds SG. Endoscopic vacuum assisted wound closure (EVAC) device to treat esophageal and gastric leaks: assessing time to proficiency and cost. Surg Endosc. 2019;33:3970-3975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Jung CFM, Müller-Dornieden A, Gaedcke J, Kunsch S, Gromski MA, Biggemann L, Seif Amir Hosseini A, Ghadimi M, Ellenrieder V, Wedi E. Impact of Endoscopic Vacuum Therapy with Low Negative Pressure for Esophageal Perforations and Postoperative Anastomotic Esophageal Leaks. Digestion. 2021;102:469-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Loske G. Endoscopic negative pressure therapy of the upper gastrointestinal tract. Chirurg. 2019;90:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 72. | Bludau M, Hölscher AH, Herbold T, Leers JM, Gutschow C, Fuchs H, Schröder W. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc. 2014;28:896-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 73. | Pournaras DJ, Hardwick RH, Safranek PM, Sujendran V, Bennett J, Macaulay GD, Hindmarsh A. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg. 2018;42:2507-2511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 74. | Sharp G, Steffens D, Koh CE. Evidence of negative pressure therapy for anastomotic leak: a systematic review. ANZ J Surg. 2021;91:537-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Mennigen R, Harting C, Lindner K, Vowinkel T, Rijcken E, Palmes D, Senninger N, Laukoetter MG. Comparison of Endoscopic Vacuum Therapy Versus Stent for Anastomotic Leak After Esophagectomy. J Gastrointest Surg. 2015;19:1229-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 76. | Zhang CC, Liesenfeld L, Klotz R, Koschny R, Rupp C, Schmidt T, Diener MK, Müller-Stich BP, Hackert T, Sauer P, Büchler MW, Schaible A. Feasibility, effectiveness, and safety of endoscopic vacuum therapy for intrathoracic anastomotic leakage following transthoracic esophageal resection. BMC Gastroenterol. 2021;21:72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Jung DH, Yun HR, Lee SJ, Kim NW, Huh CW. Endoscopic Vacuum Therapy in Patients with Transmural Defects of the Upper Gastrointestinal Tract: A Systematic Review with Meta-Analysis. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 78. | Tavares G, Tustumi F, Tristão LS, Bernardo WM. Endoscopic vacuum therapy for anastomotic leak in esophagectomy and total gastrectomy: a systematic review and meta-analysis. Dis Esophagus. 2021;34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Hayami M, Klevebro F, Tsekrekos A, Samola Winnberg J, Kamiya S, Rouvelas I, Nilsson M, Lindblad M. Endoscopic vacuum therapy for anastomotic leak after esophagectomy: a single-center's early experience. Dis Esophagus. 2021;34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P, Fockens P; CLIPPER Study Group. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2012;10:603-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 81. | Infante M, Valente M, Andreani S, Catanese C, Dal Fante M, Pizzetti P, Giudice G, Basilico M, Spinelli P, Ravasi G. Conservative management of esophageal leaks by transluminal endoscopic drainage of the mediastinum or pleural space. Surgery. 1996;119:46-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Evrard S, Le Moine O, Lazaraki G, Dormann A, El Nakadi I, Devière J. Self-expanding plastic stents for benign esophageal lesions. Gastrointest Endosc. 2004;60:894-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 83. | Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259:852-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 84. | Kamarajah SK, Bundred J, Spence G, Kennedy A, Dasari BVM, Griffiths EA. Critical Appraisal of the Impact of Oesophageal Stents in the Management of Oesophageal Anastomotic Leaks and Benign Oesophageal Perforations: An Updated Systematic Review. World J Surg. 2020;44:1173-1189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Schweigert M, Dubecz A, Stadlhuber RJ, Muschweck H, Stein HJ. Risk of stent-related aortic erosion after endoscopic stent insertion for intrathoracic anastomotic leaks after esophagectomy. Ann Thorac Surg. 2011;92:513-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Aryaie AH, Singer JL, Fayezizadeh M, Lash J, Marks JM. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surg Endosc. 2017;31:612-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | do Monte Junior ES, de Moura DTH, Ribeiro IB, Hathorn KE, Farias GFA, Turiani CV, Medeiros FS, Bernardo WM, de Moura EGH. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: Systematic review and meta-analysis. Dig Endosc. 2021;33:892-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Rausa E, Asti E, Aiolfi A, Bianco F, Bonitta G, Bonavina L. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus. 2018;31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 89. | Archid R, Bazerbachi F, Abu Dayyeh BK, Hönes F, Ahmad SJS, Thiel K, Nadiradze G, Königsrainer A, Wichmann D. Endoscopic Negative Pressure Therapy (ENPT) Is Superior to Stent Therapy for Staple Line Leak After Sleeve Gastrectomy: a Single-Center Cohort Study. Obes Surg. 2021;31:2511-2519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jürgensen C, Schreiber S, Becker T, Hampe J. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc. 2013;27:3883-3890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 91. | Berlth F, Bludau M, Plum PS, Herbold T, Christ H, Alakus H, Kleinert R, Bruns CJ, Hölscher AH, Chon SH. Self-Expanding Metal Stents Versus Endoscopic Vacuum Therapy in Anastomotic Leak Treatment After Oncologic Gastroesophageal Surgery. J Gastrointest Surg. 2019;23:67-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 92. | Tachezy M, Chon SH, Rieck I, Kantowski M, Christ H, Karstens K, Gebauer F, Goeser T, Rösch T, Izbicki JR, Bruns CJ. Endoscopic vacuum therapy versus stent treatment of esophageal anastomotic leaks (ESOLEAK): study protocol for a prospective randomized phase 2 trial. Trials. 2021;22:377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Gubler C, Vetter D, Schmidt HM, Müller PC, Morell B, Raptis D, Gutschow CA. Preemptive endoluminal vacuum therapy to reduce anastomotic leakage after esophagectomy: a game-changing approach? Dis Esophagus. 2019;32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Neumann PA, Mennigen R, Palmes D, Senninger N, Vowinkel T, Laukoetter MG. Pre-emptive endoscopic vacuum therapy for treatment of anastomotic ischemia after esophageal resections. Endoscopy. 2017;49:498-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 95. | Müller PC, Morell B, Vetter D, Raptis DA, Kapp JR, Gubler C, Gutschow CA. Preemptive Endoluminal Vacuum Therapy to Reduce Morbidity After Minimally Invasive Ivor Lewis Esophagectomy: Including a Novel Grading System for Postoperative Endoscopic Assessment of GI-Anastomoses. Ann Surg. 2021;274:751-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Yasuda JL, Svetanoff WJ, Staffa SJ, Zendejas B, Hamilton TE, Jennings RW, Ngo PD, Jason Smithers C, Manfredi MA. Prophylactic negative vacuum therapy of high-risk esophageal anastomoses in pediatric patients. J Pediatr Surg. 2021;56:944-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |