Published online Oct 16, 2021. doi: 10.4253/wjge.v13.i10.491
Peer-review started: March 6, 2021
First decision: July 3, 2021
Revised: July 26, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: October 16, 2021
In recent years, with the growing availability of image-enhanced gastrointestinal endoscopy, gastroenterologists have contributed to the early detection of pharyngeal squamous cell carcinomas (SCC).
To clarify the clinical characteristics of pharyngeal SCCs detected by gastro
This is a retrospective cohort study conducted in a single-center, a university hospital in Japan. We retrospectively assessed the clinical records of 522 consecutive patients with oropharyngeal or hypopharyngeal SCC who were examined in our hospital between 2011 and 2018. The lesions were classified into two groups: Group GE (detected by gastrointestinal endoscopy) and Group non-GE (detected by means other than gastrointestinal endoscopy). The clinical characteristics were compared between the two groups. Continuous data were compared using the Mann–Whitney U test. Pearson’s χ2 test or Fisher's exact test was used to analyze the categorical data and compare proportions. The Kaplan–Meier method was used to estimate the cumulative patient survival rates.
In our study group, the median age was 65 years and 474 patients (90.8%) were male. One hundred and ninety-six cases (37.5%) involved the oropharynx and 326 cases (62.5%) involved the hypopharynx. Three hundred and ninety-five cases (75.7%) had some symptoms at the time of diagnosis. One hundred and forty-five (27.8%) cases had concurrent ESCC or a history of ESCC. One hundred and sixty-four (31.4%) cases were detected by gastrointestinal endoscopy and classified as Group GE. The proportions of asymptomatic cases, cTis-1 cases and cases with no lymph node metastasis were significantly higher in Group GE than Group non-GE (61.6% vs 7.3%, P < 0.001, 32.9% vs 12.0%, P < 0.001 and 69.5% vs 19.0%, P < 0.001). Endoscopic laryngo-pharyngeal surgery or endoscopic submucosal dissection were performed in only 0.6% of the lesions in Group non-GE but in 21.3% of the lesions in Group GE (P < 0.001). Overall survival was significantly longer in Group GE than in Group non-GE (P = 0.018). The 2-year and 4-year survival rates were 82.5% and 70.7% in Group GE, and 71.5% and 59.0% in Group non-GE, respectively.
Gastrointestinal endoscopy plays an important role in the early detection and improving the prognosis of pharyngeal SCCs.
Core Tip: This is the first study to explore the detection modality of oropharyngeal and hypopharyngeal squamous cell carcinomas (SCC). In this study, 31.4% of pharyngeal SCCs (15.4% of oropharyngeal SCCs and 42.3% of hypopharyngeal SCCs) were detected by gastrointestinal endoscopy. The clinical characteristics of the lesions detected by gastrointestinal endoscopy include a higher proportion of asymptomatic cases, cTis-1 cases, cases with no lymph node metastasis and cases treated by endoscopic laryngo-pharyngeal surgery/endoscopic submucosal dissection, leading to a better prognosis. This study highlights the important role of gastrointestinal endoscopy in the early detection and treatment of SCC in the otolaryngology field.
- Citation: Miyamoto H, Naoe H, Morinaga J, Sakisaka K, Tayama S, Matsuno K, Gushima R, Tateyama M, Shono T, Imuta M, Miyamaru S, Murakami D, Orita Y, Tanaka Y. Clinical impact of gastrointestinal endoscopy on the early detection of pharyngeal squamous cell carcinoma: A retrospective cohort study. World J Gastrointest Endosc 2021; 13(10): 491-501
- URL: https://www.wjgnet.com/1948-5190/full/v13/i10/491.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i10.491
The pharynx is the most common site of head and neck cancer and, because pharyngeal cancers are often diagnosed at an advanced stage, the prognosis is poor[1-3]. Standard surgical resection or chemoradiotherapy (CRT) for advanced pharyngeal cancer lesions may severely reduce the patient’s quality of life, with disorders of swallowing and speech function. Similar to other gastrointestinal tumors, superficial pharyngeal cancer can be treated by minimally invasive endoscopic resection that preserves organ function[4-6]. Therefore, strategies for the detection of pharyngeal cancer at an early stage and treatment with endoscopy, including endoscopic submucosal dissection (ESD) and endoscopic laryngo-pharyngeal surgery (ELPS), are crucial for preserving the quality of life and improving prognosis.
In recent years, image-enhanced endoscopy (IEE) systems, including narrow-band imaging (NBI) and blue laser imaging, have been reported to be useful for the early detection of cancer in the pharynx and esophagus[7,8]. Patients with head-and-neck squamous cell cancer (HNSCC) or esophageal squamous cell carcinoma (SCC) (ESCC) have a high risk of synchronous and metachronous SCCs, which has been recognized as the field cancerization phenomenon[9,10]. Therefore, patients with present or previous HNSCC or ESCC require careful endoscopic observation of the pharynx with IEE[11,12]. In general, pharyngeal cancers have been most often detected by otolaryngologists using rhino-laryngoscopy. Recently, many superficial pharyngeal cancers have been discovered by gastroenterologists, with the growing availability of IEE in gastrointestinal endoscopy. However, few studies have shown how much gastroenterologists contribute to the detection and treatment of pharyngeal cancer.
Previously, we investigated the modalities of detection of superficial hypo
In this retrospective study, we assessed the clinical records of consecutive patients with oropharyngeal or hypopharyngeal SCC who underwent a detailed examination, including definitive diagnosis by pathologists and staging based on the TNM classification, in our hospital between January 2011 and December 2018. The first lesion detected during the study period was included in the analysis. If multiple lesions were detected at the same time, the largest lesion was included. We excluded patients who had undergone prior treatment of pharyngeal cancer at another hospital and/or had unspecified details of detection modality. The following data were reviewed retrospectively: The physician who detected the primary lesion (gastroenterologist, otolaryngologist, dentist, general physician), indication for examination of the pharynx, clinical manifestation, age at incidence, sex, tumor location, primary treatment, TNM classification[14], past history of ESCC, patient vital status (alive, deceased, lost to follow-up) and follow-up time.
We defined those with lesions detected by gastrointestinal endoscopy as Group GE and those with lesions detected by means other than gastrointestinal endoscopy (rhino-laryngoscopy or direct visualization by otolaryngologists, dentists and general physicians) as Group non-GE.
The oropharynx was divided into the following four subsites: (1) Anterior wall: Base of tongue; (2) Superior wall: Inferior surface of soft palate and uvula; (3) Lateral wall: Tonsil, tonsillar fossa, and pillars; and (4) Posterior wall. The hypopharynx was divided into the following three subsites: (1) Pyriform sinus; (2) Posterior wall; and (3) Post-cricoid region. We defined the symptomatic group as patients with any one of the following conditions: Sore throat, painful swallowing, pharyngeal discomfort, bleeding, swelling of cervical lymph nodes or hoarseness.
We evaluated the proportion of Group GE among all pharyngeal cancer, the clinical differences between Group GE and Group non-GE, and the trends in proportion of Group GE.
This study was approved by the ethical committee of our hospital and performed in accordance with the ethical principles associated with the Declaration of Helsinki.
Patients and the public were not involved in the design, conduct, reporting, or dissemination of plans of the research.
Continuous data were compared using the Mann–Whitney U test. Pearson’s χ2 test or Fisher's exact test were used to analyze the categorical data and compare proportions. The survival rates of patients were plotted using Kaplan–Meier curves, and the difference was evaluated using the log rank test. Cox regression analysis was used to estimate the hazard ratio and to calculate the 95% confidence interval. SPSS version 21.0 (IBM Corporation, Armonk, NY, United States) was used for all statistical analyses. P values < 0.05 (two-sided) denoted statistically significant differences. The statistical methods of this study were reviewed by our expert biostatistician, Jun Morinaga, MD.
From January 2011 to December 2018, 563 lesions (oropharyngeal and hypopharyngeal SCCs) in 535 patients were examined in our hospital. Of those, 41 lesions and 13 patients were excluded (28 lesions in 26 patients were excluded due to multiple primary lesions; seven lesions in seven patients had been treated at another hospital; and the details of the detection process were not specified for six lesions in six patients). Hence, a total of 522 lesions in 522 patients were enrolled in this study. The median duration of follow-up was 25.8 mo.
The characteristics of the study population are listed in Table 1. The median age was 65 years and 474 patients (90.8%) were male. One hundred and ninety-six cases (37.5%) were in the oropharynx and 326 cases (62.5%) were in the hypopharynx. Three hundred and ninety-five cases (75.7%) had symptoms of some kind at the time of diagnosis. The most common reason for the examination was the investigation of symptoms (71.1%). One hundred and sixty-four (31.4%) cases were detected by gastrointestinal endoscopy (Group GE). Among 358 cases detected other than by gastrointestinal endoscopy (Group non-GE), almost all lesions were detected by otolaryngologists (341 lesions) and the remainder were detected by dentists (14 lesions) and general physicians (three lesions). One hundred and forty-five (27.8%) cases had concurrent ESCC or a history of ESCC.
| n = 522 | |
| Sex, male/female | 474 (90.8%)/48 |
| Age, median, yr | 65 (37-92) |
| Location | |
| Oropharynx | 196 (37.5%) |
| Hypopharynx | 326 (62.5%) |
| Symptomatic/Asymptomatic | 395 (75.7%)/127 |
| Indication for examination | |
| Investigation of symptoms | 371 (71.1%) |
| Incidental EGD | 47 (9.0%) |
| f/u or diagnostic work-up of ESCC | 66 (12.6%) |
| f/u or diagnostic work-up of HN | 17 (3.3%) |
| Incidental dental check | 7 (1.3%) |
| Other | 14 (2.7%) |
| Detected by GE/non-GE | 164 (31.4%)/358 |
| cTis-1/2/3/4 | 97 (18.6%)/177/102/146 |
| cN -/+ | 182 (34.9%)/340 |
| cM -/+ | 504 (96.6%)/18 |
| Concurrent or history of ESCC y/n | 145 (27.8%)/377 |
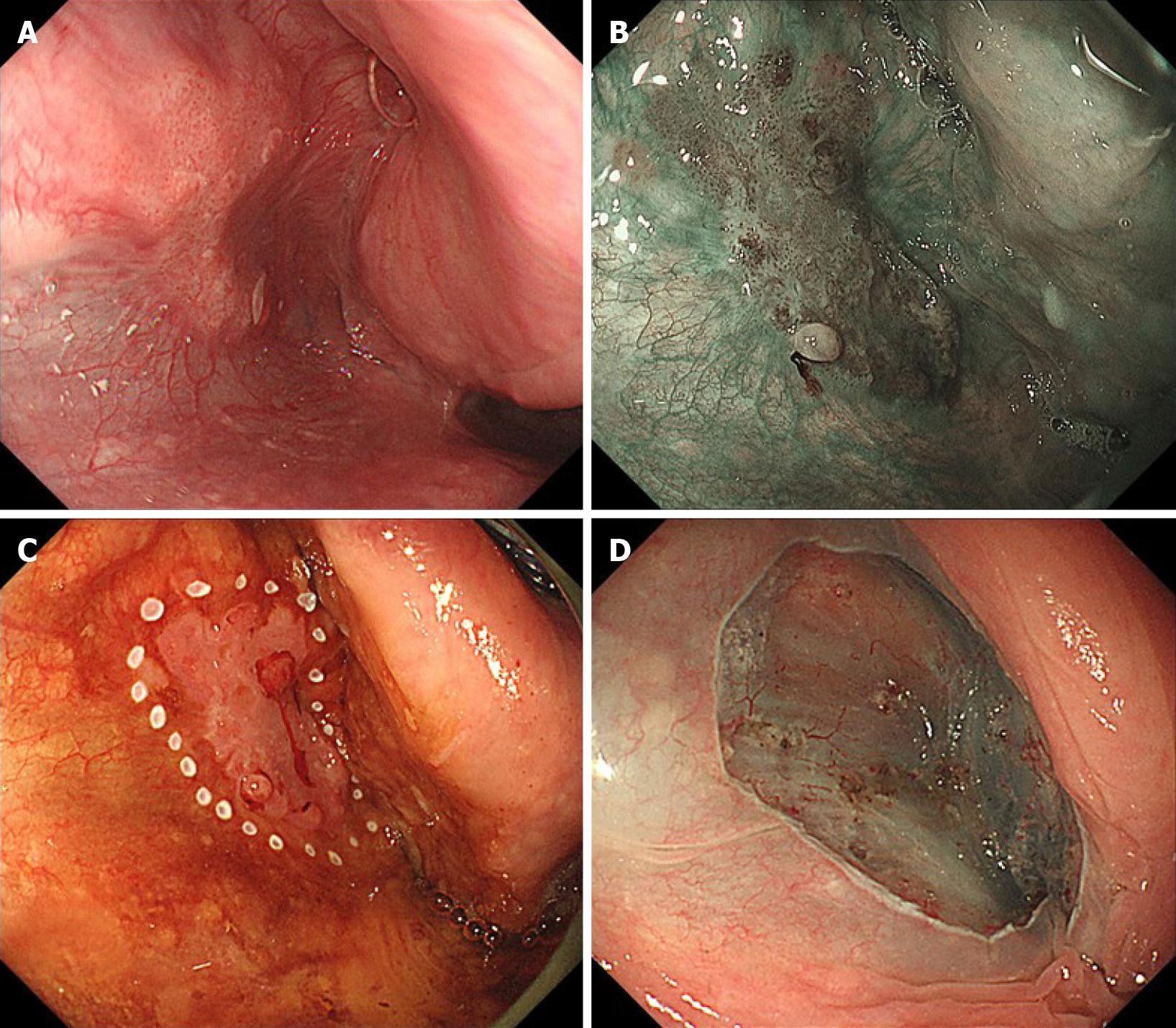
A comparison between Group GE and Group non-GE is shown in Table 2. There were no significant differences in sex or age. The proportion of symptomatic cases was significantly lower in Group GE (38.4% vs 92.7%, P < 0.001). The common reasons for the examination were follow-up or diagnostic work-up for ESCC (39.0%), incidental esophago-gastro-duodenoscopy (EGD) (28.7%) and investigation of symptoms (28.0%) in Group GE and investigation of symptoms (90.8%) in Group non-GE. Incidental EGD included screening for gastric cancer (46.8%), surveillance of gastric cancer (10.6%), investigation of abdominal symptom (10.6%), and others (31.9%). As for the primary site, the proportion of oropharynx lesions was significantly lower in Group GE than Group non-GE (15.9% vs 47.5%, P < 0.001). The proportion of lesions with concurrent or a history of ESCC was significantly higher in Group GE than Group non-GE (51.2% vs 17.0%, P < 0.001). The proportions of cTis-1 cases and cases with no lymph node metastasis were significantly higher in Group GE than Group non-GE (32.9% vs 12.0%, P < 0.001 and 69.5% vs 19.0%, P < 0.001). Meanwhile, there were no significant differences in the proportion of cases with distant metastases. As for the modality of treatment, ELSP/ESD was performed in only 0.6% of cases in Group non-GE, while 21.3% of cases in Group GE were treated with ELPS/ESD (P < 0.001). We showed a case of T1 hypopharyngeal cancer located in the left pyriform sinus and detected by gastrointestinal endoscopy with NBI (Figure 1). Under general anesthesia, en bloc resection by ESD was successfully completed.
| Group GE n = 164 | Group non-GE n = 358 | P value | |
| Sex, male | 153 (93.3%) | 321 (89.7%) | 0.197 |
| Age, median, yr | 68 (42–90) | 67 (37–92) | 0.278 |
| Asymptomatic/Symptomatic | 101 (61.6%)/63 (38.4%) | 26 (7.3%)/332 (92.7%) | < 0.001 |
| Indication for examination | < 0.001 | ||
| Investigation of symptoms | 46 (28.0%) | 325 (90.8%) | |
| Incidental EGD | 47 (28.7%) | 0 | |
| f/u or diagnostic work-up of ESCC | 64 (39.0%) | 2 (0.6%) | |
| f/u or diagnostic work-up of HN | 7 (4.3%) | 10 (2.8%) | |
| Incidental dental check | 0 | 7 (2.0%) | |
| Other | 0 | 14 (3.9%) | |
| Location oropharynx/hypopharynx | 26 (15.9 %)/138 (84.1%) | 170 (47.5%)/188 (52.5%) | < 0.001 |
| History or concurrent of ESCC, y/n | 84 (51.2%)/80 | 61 (17.0%)/297 | < 0.001 |
| cTis-1/cT2-4 | 54 (32.9%)/110 | 43 (12.0%)/315 | < 0.001 |
| cN -/+ | 114 (69.5%)/50 | 68 (19.0%)/290 | < 0.001 |
| cM -/+ | 161 (98.2%)/3 | 343 (95.8%)/15 | 0.205 |
| Treatment | |||
| ELPS/ESD | 35 (21.3%) | 2 (0.6%) | < 0.001 |
| Non-ELPS/ESD | 129 (78.7%) | 356 (99.4%) | |
| Surgery | 23 (14.0%) | 79 (22.1%) | |
| RT/CRT | 84 (51.2%) | 212 (59.2%) | |
| Chemotherapy | 5 (3.0%) | 13 (3.6%) | |
| BSC | 9 (5.5%) | 40 (11.2%) | |
| Unknown | 8 (4.9%) | 12 (3.4%) |
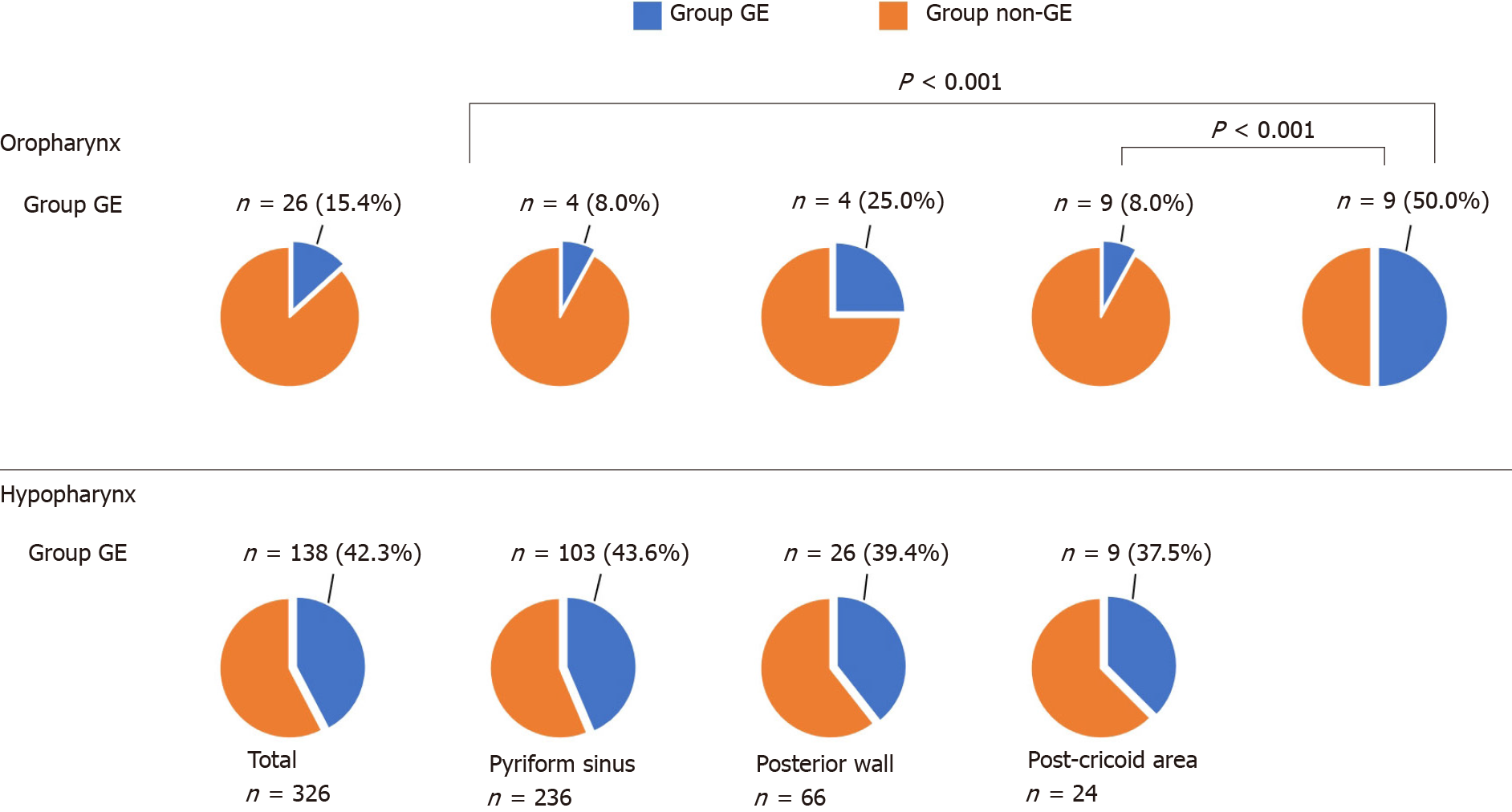
Figure 2 shows the subsite of primary lesions and the proportion of Group GE by subsite. The proportions of Group GE in the oropharynx and hypopharynx were 15.4% and 42.3%, respectively. In the oropharynx, the proportions of Group GE in the anterior (8.0%) and lateral wall (8.5%) were significantly lower than the posterior wall (50.0%). On the other hand, in the hypopharynx, there was no significant difference in the proportion of Group GE by subsite.
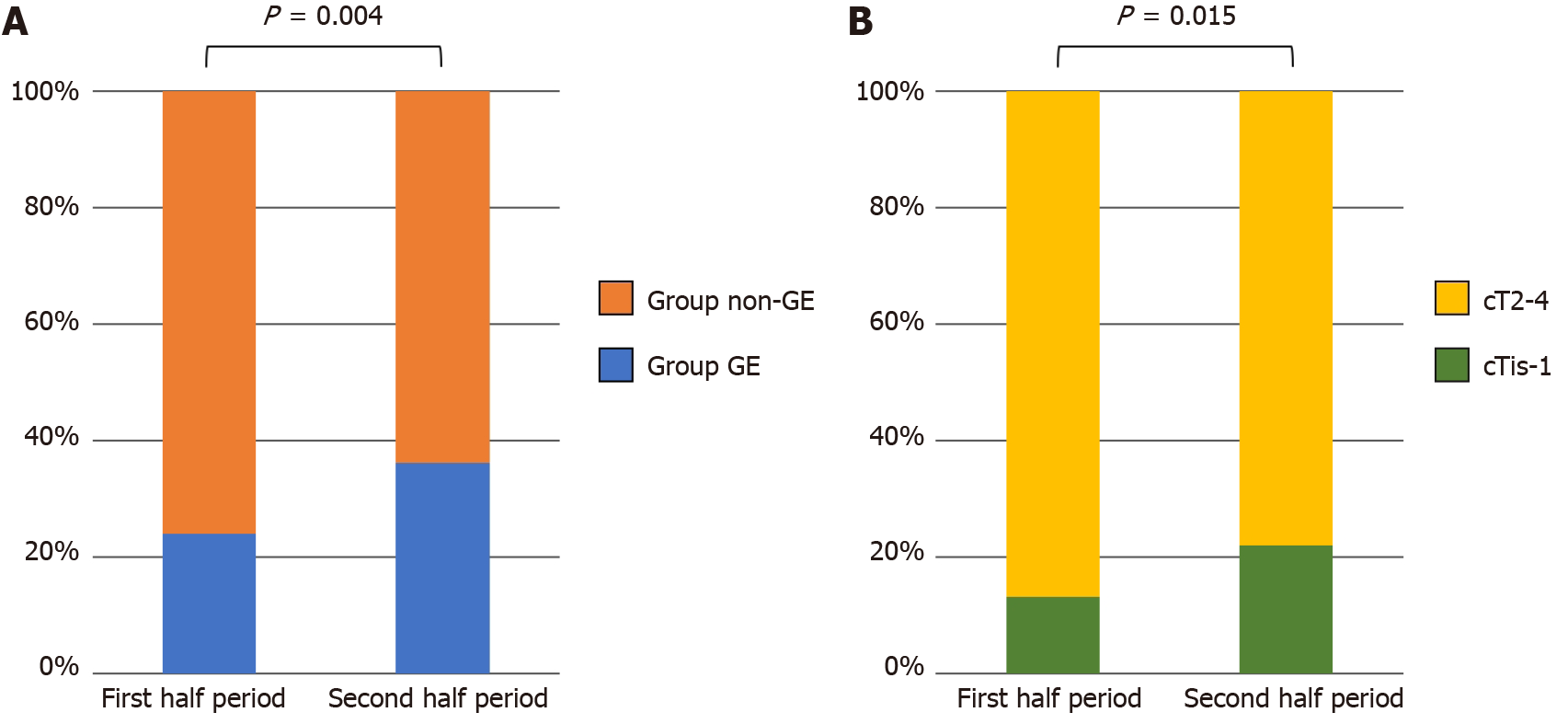
Figure 3A shows a comparison of the proportion of Group GE between the first and second half periods (2011–2014 and 2015–2018). The proportion of Group GE was significantly larger in the second half period (24.0% vs 36.2%, P = 0.004). Consistent with this tendency, the proportion of cTis-1 lesions was significantly higher in the second half period (13.2% vs 22.0%, P = 0.015) (Figure 3B).
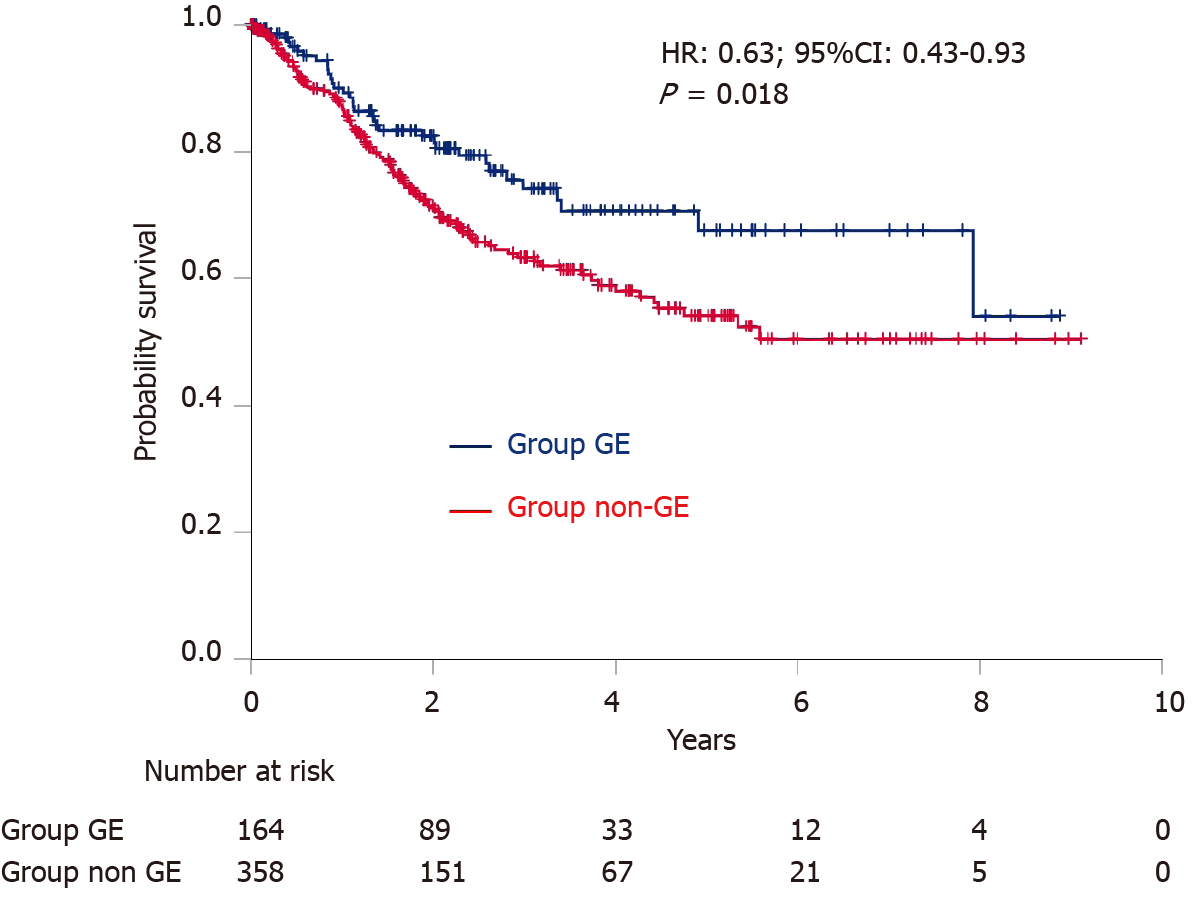
Kaplan–Meier curves of survival are shown in Figure 4. Overall survival was significantly longer in Group GE than in Group non-GE (HR: 0.63; 95%CI: 0.43-0.93; P = 0.018). The 2-year and 4-year survival rates were 82.5% and 70.7% in Group GE, and 71.5% and 59.0% in Group non-GE, respectively.
This study investigated the impact of gastrointestinal endoscopy on the detection of pharyngeal SCC. Of total 522 lesions, 164 (31.4%) in Group GE had a higher proportion of asymptomatic cases, cTis-1 cases, cases with no lymph node metastasis and cases treated by ELPS/ESD than Group non-GE, leading to a better prognosis. To the best of our knowledge, this is the first study to explore the detection modality of oropharyngeal and hypopharyngeal SCC in a large number of cases.
Until the advent of NBI, gastrointestinal endoscopists were unable to observe the pharynx in detail, thereby posing a challenge to the detection of pharyngeal cancer using gastrointestinal endoscopy. In 2010, the usefulness of NBI for the early detection of cancer in the pharynx was reported. Muto et al[7] conducted a multicenter, prospective, randomized controlled trial; 320 patients with ESCC were randomly assigned to primary white light imaging (WLI) followed by NBI or primary NBI followed by WLI in a back-to-back fashion. They reported that the sensitivity and accuracy were significantly higher in the NBI-first group than the WLI-first group in both the head and neck region and the esophagus (100% vs 7.7%; P < 0.001 for sensitivity, 85.7% vs 62.9%; P = 0.02 for accuracy, respectively). In a study of 424 consecutive patients subjected to surveillance endoscopy who had previously undergone CRT and/or surgery for esophageal SCC, Nonaka et al[15] reported that the detection rate for pharyngeal cancer was significantly higher when using NBI endoscopy with magnification (10.9%) compared with conventional endoscopy (1.2%) (P < 0.0001). Following these reports, careful endoscopic observation of the pharynx with IEE for patients with ESCCs became gradually popular among Japanese gastroenterologists[11,12,16,17]. These observations revealed the usefulness of gastrointestinal endoscopy for the detection of pharyngeal cancer among patients with esophageal SCC. However, the proportion and clinical characteristics of the lesions detected by gastrointestinal endoscopy among patients with pharyngeal cancer remained unclear. The advantage of the present study is to elucidate the clinical characteristics of pharyngeal SCCs detected by gastrointestinal endoscopy.
A recent systematic review and meta-analysis revealed that the prevalence of head and neck second primary tumors in patients with ESCC was 6.7%, and 60% of all head and neck second primary tumors were located in the hypopharynx, with 18% in the oropharynx[18]. In our study, the percentage of concurrent ESCC or with a history of ESCC was 27.8%. Considering these data, the careful endoscopic observation of the pharynx of patients with present or previous ESCC is efficient, but it is insufficient because 70.7% of pharyngeal SCCs were not relevant to ESCCs. In Group non-GE, 92.7% of cases were symptomatic and only 0.6% of cases were treated by ELPS/ESD. The problem appears to be that patients do not visit hospital and receive an otolaryngology examination unless the cancer has progressed to a symptomatic stage. On the other hand, in Group GE, only 38.4% of cases were symptomatic and the proportion of cases treated by ELPS/ESD was significantly higher (21.3%) than Group non-GE. It is important to detect pharyngeal SCCs with gastrointestinal endoscopy while patients remain asymptomatic for further improvement in prognosis and preservation of function. On this basis, we should not pass through the pharynx without due caution in patients with risk factors (e.g., smoking, alcohol consumption), even if they have no history of ESCC and no symptoms. In the present study, pharyngeal cancer was detected in hospitals, as well as clinics and health examination centers. Moreover, the numbers of lesions detected by gastrointestinal endoscopy have been increasing (Figure 3). Furthermore, due to advances in endoscopic treatment, we have been able to remove superficial pharyngeal lesions by ELPS/ESD, without impairment of pharyngeal function[19,20]. We emphasize that gastrointestinal endoscopists can improve the prognosis of patients with pharyngeal cancer by careful observation of the pharynx in routine clinical practice, and should take a more active role both in the detection and treatment of this type of cancer.
In our study, the proportions of lesions in the anterior and lateral wall of oropharynx were extremely low in Group GE (7.8% and 8.5%, respectively). One of the reasons is that the lateral and anterior walls of the oropharynx are anatomically difficult to observe using transoral endoscopy, so even advanced cancer may be easily missed if the endoscope is passed too quickly through the oropharynx[21]. The other cause is possibly related to human papillomavirus (HPV). HPV infection has been identified as a risk factor for oropharyngeal SCCs, especially involving the tonsils and base of the tongue[22]. Because HPV infects the basal layer of the tonsillar crypt, cancer arises from the deeper areas and is not always exposed at the luminal surface at an early stage. Thus, endoscopic diagnosis tends to be difficult compared to HPV-unrelated pharyngeal SCCs[23]. In this study, we were not able to show the percentage of HPV-related cancer due to insufficient data. Although early pharyngeal cancers were detected mostly by gastroenterologists, considering that some lesions are difficult to detect with gastrointestinal endoscopy, pharyngeal examination conducted by otolaryngologists and gastroenterologists in cooperation will be required for further improvement of cancer detection.
There were some limitations in the present study. Firstly, it is a retrospective review of hospital records from a single center. Therefore, the history of gastrointestinal endoscopic examination was uncertain in Group non-GE and we could not determine how often gastroenterologists had missed the pharyngeal lesions. Furthermore, we could not survey the experiences of individual physicians or the accessibility to gastrointestinal endoscopy and otolaryngology services in individual residential areas. In the future, a prospective study should be designed to address this subject. Secondly, there was referral filter bias because almost all ELPS/ESD cases were treated in our hospital in Kumamoto prefecture. This would increase the proportion of Group GE. However, as our hospital is the only university hospital in Kumamoto prefecture, most advanced cases which required surgery or CRT were referred here, as well as ELPS/ESD cases, and we consider our data represent the current situation in Kumamoto prefecture.
Gastrointestinal endoscopy is playing an increasingly important role in the detection of pharyngeal SCCs, considering that 31.4% of all cases and almost all asymptomatic cases were detected by gastrointestinal endoscopy. For preserving the quality of life and improving the prognosis of pharyngeal SCCs, it is important to detect the lesions using gastrointestinal endoscopy, while they are asymptomatic.
Recently, many pharyngeal cancers have been discovered by gastroenterologists, with the growing availability of image enhanced endoscopy in gastrointestinal endoscopy. However, few studies have shown how much gastroenterologists contribute to the detection and treatment of pharyngeal cancer. In particular, the details of the lesions detected by the gastrointestinal endoscopy are unknown.
To highlight that gastrointestinal endoscopists should take a more active role both in the detection and treatment of pharyngeal cancer.
To clarify the importance of gastrointestinal endoscopy in detection and treatment of pharyngeal cancer.
In this retrospective cohort study, the authors assessed the clinical records of consecutive 522 patients with oropharyngeal or hypopharyngeal cancer in our hospital between January 2011 and December 2018. The lesions were classified into two groups: Group GE (detected by gastrointestinal endoscopy) and Group non-GE (detected by means other than gastrointestinal endoscopy), and the clinical characteristics were compared between the two groups.
Of total 522 lesions, 164 (31.4%) in Group GE had a higher proportion of asymptomatic cases (61.6% vs 7.3%, P < 0.001), cTis-1 cases (32.9% vs 12.0%, P < 0.001), cases with no lymph node metastasis (69.5% vs 19.0%, P < 0.001) and cases treated by endoscopic laryngo-pharyngeal surgery/endoscopic submucosal dissection (21.3% vs 0.6%, P < 0.001) than Group non-GE, leading to a better prognosis.
To the best of our knowledge, this is the first study to explore the detection modality of oropharyngeal and hypopharyngeal squamous cell carcinomas (SCC) in a large number of cases. Gastrointestinal endoscopy plays an important role in the early detection and improving the prognosis of pharyngeal SCCs.
In the future, a multicenter prospective study should be designed in a set up where equal accessibility to gastrointestinal endoscopy and otolaryngology services is available.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Koritala T S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Wahlberg PC, Andersson KE, Biörklund AT, Möller TR. Carcinoma of the hypopharynx: analysis of incidence and survival in Sweden over a 30-year period. Head Neck. 1998;20:714-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Eckel HE, Staar S, Volling P, Sittel C, Damm M, Jungehuelsing M. Surgical treatment for hypopharynx carcinoma: feasibility, mortality, and results. Otolaryngol Head Neck Surg. 2001;124:561-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Rikitake R, Ando M, Saito Y, Yoshimoto S, Yamasoba T, Higashi T. Current status of superficial pharyngeal squamous cell carcinoma in Japan. Int J Clin Oncol. 2017;22:826-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Hanaoka N, Ishihara R, Takeuchi Y, Suzuki M, Uemura H, Fujii T, Yoshino K, Uedo N, Higashino K, Ohta T, Kanzaki H, Hanafusa M, Nagai K, Matsui F, Iishi H, Tatsuta M, Tomita Y. Clinical outcomes of endoscopic mucosal resection and endoscopic submucosal dissection as a transoral treatment for superficial pharyngeal cancer. Head Neck. 2013;35:1248-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Hanaoka N, Ishihara R, Takeuchi Y, Suzuki M, Otozai S, Kida K, Yoshii T, Fujii T, Yoshino K, Sugawa T, Kitamura K, Kanemura R, Koike R, Uedo N, Higashino K, Akasaka T, Yamashina T, Kanesaka T, Matsuura N, Aoi K, Yamasaki Y, Hamada K, Iishi H, Tomita Y. Endoscopic submucosal dissection as minimally invasive treatment for superficial pharyngeal cancer: a phase II study (with video). Gastrointest Endosc. 2015;82:1002-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Koritala T, Zolotarevsky E, Bartley AN, Ellis CD, Krolikowski JA, Burton J, Gunaratnam NT. Efficacy and safety of the band and slough technique for endoscopic therapy of nonampullary duodenal adenomas: a case series. Gastrointest Endosc. 2015;81:985-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, Inoue H, Ishikawa H, Ochiai A, Shimoda T, Watanabe H, Tajiri H, Saito D. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 476] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 8. | Tomie A, Dohi O, Yagi N, Kitae H, Majima A, Horii Y, Kitaichi T, Onozawa Y, Suzuki K, Kimura-Tsuchiya R, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Itoh Y. Blue Laser Imaging-Bright Improves Endoscopic Recognition of Superficial Esophageal Squamous Cell Carcinoma. Gastroenterol Res Pract. 2016;2016:6140854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 10. | Katada C, Yokoyama T, Yano T, Kaneko K, Oda I, Shimizu Y, Doyama H, Koike T, Takizawa K, Hirao M, Okada H, Yoshii T, Konishi K, Yamanouchi T, Tsuda T, Omori T, Kobayashi N, Shimoda T, Ochiai A, Amanuma Y, Ohashi S, Matsuda T, Ishikawa H, Yokoyama A, Muto M. Alcohol Consumption and Multiple Dysplastic Lesions Increase Risk of Squamous Cell Carcinoma in the Esophagus, Head, and Neck. Gastroenterology. 2016;151:860-869.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 11. | Morimoto H, Yano T, Yoda Y, Oono Y, Ikematsu H, Hayashi R, Ohtsu A, Kaneko K. Clinical impact of surveillance for head and neck cancer in patients with esophageal squamous cell carcinoma. World J Gastroenterol. 2017;23:1051-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Kato M, Ishihara R, Hamada K, Tonai Y, Yamasaki Y, Matsuura N, Kanesaka T, Yamamoto S, Akasaka T, Hanaoka N, Takeuchi Y, Higashino K, Uedo N, Iishi H. Endoscopic surveillance of head and neck cancer in patients with esophageal squamous cell carcinoma. Endosc Int Open. 2016;4:E752-E755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Kumai Y, Shono T, Waki K, Murakami D, Miyamaru S, Sasaki Y, Orita Y. Detection of hypopharyngeal cancer (Tis, T1 and T2) by ENT physicians vs gastrointestinal endoscopists. Auris Nasus Larynx. 2020;47:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Amin MB, Edge S. AJCC Cancer Staging Manual, 8th ed. Springer International Publishing, 2017: XVII, 1032. [Cited in This Article: ] |
| 15. | Nonaka S, Saito Y, Oda I, Kozu T, Saito D. Narrow-band imaging endoscopy with magnification is useful for detecting metachronous superficial pharyngeal cancer in patients with esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2010;25:264-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Katada C, Tanabe S, Koizumi W, Higuchi K, Sasaki T, Azuma M, Katada N, Masaki T, Nakayama M, Okamoto M, Muto M. Narrow band imaging for detecting superficial squamous cell carcinoma of the head and neck in patients with esophageal squamous cell carcinoma. Endoscopy. 2010;42:185-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Katada C, Muto M, Tanabe S, Higuchi K, Sasaki T, Azuma M, Ishido K, Masaki T, Nakayama M, Okamoto M, Koizumi W. Surveillance after endoscopic mucosal resection or endoscopic submucosal dissection for esophageal squamous cell carcinoma. Dig Endosc. 2013;25 Suppl 1:39-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | van de Ven S, Bugter O, Hardillo JA, Bruno MJ, Baatenburg de Jong RJ, Koch AD. Screening for head and neck second primary tumors in patients with esophageal squamous cell cancer: A systematic review and meta-analysis. United European Gastroenterol J. 2019;7:1304-1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Iizuka T, Kikuchi D, Hoteya S, Nakamura M, Yamashita S, Mitani T, Takeda H, Yahagi N. Clinical advantage of endoscopic submucosal dissection over endoscopic mucosal resection for early mesopharyngeal and hypopharyngeal cancers. Endoscopy. 2011;43:839-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Tateya I, Muto M, Morita S, Miyamoto S, Hayashi T, Funakoshi M, Aoyama I, Higuchi H, Hirano S, Kitamura M, Ishikawa S, Kishimoto Y, Morita M, Ito J. Endoscopic laryngo-pharyngeal surgery for superficial laryngo-pharyngeal cancer. Surg Endosc. 2016;30:323-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Hamada K, Ishihara R, Yamasaki Y, Akasaka T, Arao M, Iwatsubo T, Shichijo S, Matsuura N, Nakahira H, Kanesaka T, Yamamoto S, Takeuchi Y, Higashino K, Uedo N, Kawahara Y, Okada H. Transoral endoscopic examination of head and neck region. Dig Endosc. 2018;30:516-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Ramqvist T, Grün N, Dalianis T. Human papillomavirus and tonsillar and base of tongue cancer. Viruses. 2015;7:1332-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Okami K. Clinical features and treatment strategy for HPV-related oropharyngeal cancer. Int J Clin Oncol. 2016;21:827-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |