Published online Jan 18, 2017. doi: 10.4254/wjh.v9.i2.106
Peer-review started: June 30, 2016
First decision: August 26, 2016
Revised: September 23, 2016
Accepted: December 1, 2016
Article in press: December 2, 2016
Published online: January 18, 2017
Processing time: 203 Days and 0.8 Hours
To examine patient-centered outcomes with vasopressin (AVP) use in patients with cirrhosis with catecholamine-refractory septic shock.
We conducted a single center, retrospective cohort study enrolling adult patients with cirrhosis treated for catecholamine-resistant septic shock in the intensive care unit (ICU) from March 2011 through December 2013. Other etiologies of shock were excluded. Multivariable regression models were constructed for seven and 28-d mortality comparing AVP as a second-line therapy to a group of all other vasoactive agents.
Forty-five consecutive patients with cirrhosis were treated for catecholamine-resistant septic shock; 21 received AVP while the remaining 24 received another agent [phenylephrine (10), dopamine (6), norepinephrine (4), dobutamine (2), milrinone (2)]. In general, no significant differences in baseline demographics, etiology of cirrhosis, laboratory values, vital signs or ICU mortality/severity of illness scores were observed with the exception of higher MELD scores in the AVP group (32.4, 95%CI: 28.6-36.2 vs 27.1, 95%CI: 23.6-30.6, P = 0.041). No statistically significant difference was observed in unadjusted 7-d (52.4% AVP vs 58.3% and P = 0.408) or 28-d mortality (81.0% AVP vs 87.5% non-AVP, P = 0.371). Corticosteroid administration was associated with lower 28-d mortality (HR = 0.37, 95%CI: 0.16-0.86, P = 0.021) independent of AVP use.
AVP is similar in terms of patient centered outcomes of seven and 28-d mortality, in comparison to all other vasopressors when used as a second line vasoactive agent in catecholamine resistant septic shock. Large-scale prospective study would help to refine current consensus standards and provide further support to our findings.
Core tip: Although the management of septic shock has evolved dramatically in recent decades, data regarding optimal vasopressor therapy in critically-ill patients with cirrhosis is less robust and is based largely on consensus expert opinion. We found no difference in 7-d or 28-d mortality with vasopressin use when compared to all other vasoactive agents as a second line agent in catecholamine-resistant septic shock. Further large-scale studies are needed to refine current consensus standards and provide further support to our findings.
- Citation: Myc LA, Stine JG, Chakrapani R, Kadl A, Argo CK. Vasopressin use in critically ill cirrhosis patients with catecholamine-resistant septic shock: The CVICU cohort. World J Hepatol 2017; 9(2): 106-113
- URL: https://www.wjgnet.com/1948-5182/full/v9/i2/106.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i2.106
The management of septic shock has evolved since the inception of the Surviving Sepsis Campaign and the adoption of early goal-directed therapy, with short-term mortality rates improving markedly over the past decade[1]. Improved outcomes appear to have extended to special populations as well, including patients with cirrhosis of the liver, a population in which sepsis has traditionally been characterized by extremely high mortality rates of nearly 100% in some studies, well above those of the general population which approximate 40% at 28-d[2-4]. Concurrent with the development of bundled care protocols, the incorporation of arginine vasopressin (AVP) into the management of septic shock has generated significant clinical and research interest. Based on reports of inappropriately low levels of circulating AVP coupled with apparent AVP-hypersensitivity in patients with cirrhosis and septic shock, exogenous AVP was seen as potentially restorative of both vascular tone and catecholamine-sensitivity in septic states[5-7].
Current recommendations for AVP use in managing septic shock largely derive from the published results of the Vasopressin and Septic Shock Trial (VASST) which reported no significant difference in 28-d mortality in patients with septic shock treated with vasopressin vs norepinephrine[4]. Nevertheless, the authors did report improved 28-d mortality in a pre-specified sub-group of patients with less severe septic shock as well as decreased norepinephrine requirements in patients receiving AVP, leading to the adoption of exogenous AVP use as an ungraded recommendation into the Surviving Sepsis Guidelines.
Appreciating these general recommendations, it remains unclear what role exogenous AVP may serve in patients with cirrhosis given the unique characteristics of septic shock in this population. Although low levels of AVP coinciding with AVP-vasosensitivity have been reported in patients with cirrhosis, the distinctive features of septic shock in this population including hyperdynamic circulation, relative adrenal insufficiency, blood volume sequestration in the splanchnic venous plexus, and hypothermia together with underlying thrombocytopenia and varying degrees of hepatic dysfunction introduce ambiguity as to whether the generic Surviving Sepsis guidelines ought to be applied to patients with cirrhosis[2,3,8-10]. Data regarding AVP and AVP analogue use in patients with cirrhosis and septic shock are sparse.
Recently published guidelines addressing management of critically ill patients with cirrhosis do incorporate AVP use for treatment of persistent hypotension, however this recommendation relies largely on studies of terlipressin in non-cirrhotic populations[11]. In this respect, it should be noted that only 11.3% of the patients enrolled in the VASST study had any liver disease at all. While AVP may have salient effects in this population relating to improved hemodynamics, mobilization of large splanchnic blood volume, norepinephrine sparing, and improved catecholamine resistance, potential adverse effects specific to the cirrhotic state cannot be excluded and may include acute-on-chronic liver failure, worsening thrombocytopenia and hyponatremia, and decreased cardiac output[4,12-17]. Decreased cardiac output may be particularly significant in this population, which may be more dependent on oxygen delivery for oxygen consumption[18]. Together, such hepatic, renal and hematologic effects of AVP may be disproportionately detrimental in a vulnerable cirrhotic population often characterized by baseline hyponatremia and thrombocytopenia complicating underlying hepatic dysfunction.
In this single center retrospective cohort study, we aimed to characterize 7-d and 28-d mortality outcomes of AVP use in patients with cirrhosis and catecholamine-refractory septic shock (CRSS). Secondarily, we aimed to investigate the effect of AVP on 24-h changes in important laboratory parameters including aminotransferases, total bilirubin and platelet concentrations as well as heart rate. We hypothesized that use of AVP as a second vasopressor in cirrhosis patients with catecholamine-resistant septic shock would be associated with increased mortality when compared with cirrhosis patients receiving an alternate adjunct vasoactive agent (e.g., norepinephrine, phenylephrine, dopamine).
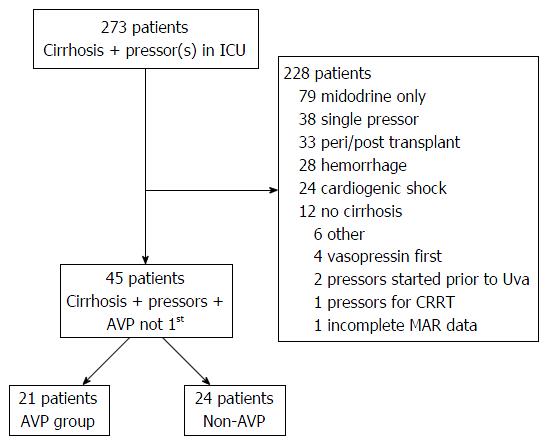
All adult patients with cirrhosis treated for CRSS shock requiring medical intensive care unit (ICU) care between March 4, 2011 and December 31, 2013 were identified through the University of Virginia Clinical Data Repository using billing and administrative codes in conjunction with data derived from medication administration reports. Cirrhosis of the liver was confirmed by direct histological examination of liver biopsy or by biochemical and imaging findings suggesting advanced liver disease with portal hypertension. Catecholamine-resistant septic shock was defined as a clinical requirement for ≥ 2 vasopressors (the first of which had to be a catecholaminergic agent) for hypotension attributable to an infectious origin on the basis of either culture data or clear clinical suspicion. Patients with cirrhosis meeting this definition of CRSS were included in our analysis. Patients with other etiologies of shock (e.g., hemorrhagic, obstructive, etc.) were excluded, as were patients who received AVP as the first vasopressor agent, patients who received vasopressors in the peri-transplant setting or for purposes of tolerating renal replacement therapy, or patients who were initiated on vasopressor therapy at an undetermined time prior to interhospital transfer to our facility (Figure 1).
Baseline patient characteristics were reviewed, including demographics, medical comorbidities (coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, diabetes, hypertension), smoking and alcohol use, etiology of liver disease with portal hypertensive complications (ascites, and hepatic encephalopathy), vital signs (heart rate, minimum mean arterial pressure, temperature, maximum respiratory rate) and laboratory values. MELD score was calculated using the standard formula: 11.2 × ln(INR) + 9.57 × ln[creatinine (mg/dL)] + 3.78 × ln[bilirubin (mg/dL)] + 6.43 with a lower limit of 1.0 for all variables[19]. ICU severity of illness variables were also collected including fraction of inspired oxygen, partial pressure of arterial carbon dioxide, partial pressure of arterial oxygen, pH, mean number of vasopressors, days on vasopressors, need for continuous renal replacement therapy, intubation, urine output over the first 24 h, new hemorrhage and new diagnosis of venous thrombosis. Illness severity scores were calculated [acute physiology and chronic health evaluation II (APACHE II), simplified acute physiology score (SAPSII), sequential organ failure assessment (SOFA)]. ICU medications were reviewed (volume of intravenous fluid, octreotide, antibiotic administration, albumin administration, proton pump inhibitor, corticosteroids and first vasopressor use). Captured outcomes included mean survival, hospital and ICU length of stay, ventilator free days, mortality (7-d, 28-d and 90-d), in-hospital mortality, in-ICU mortality and withdrawal of care. The 24-h changes in laboratory parameters (platelets, liver associated enzymes, heart rate, total bilirubin) were also extracted on the basis of the first available value of the parameter of interest available 24-48 h following vasopressor initiation.
Subjects were sorted into two groups, those patients who received AVP as the second-line agent and those patients where another vasopressor was utilized as the second-line agent. The AVP group was compared to the non-AVP group in multiple factors including baseline patient demographics, medical comorbidities, smoking and alcohol use, etiology of liver disease, portal hypertensive complications, vital signs, laboratory values, severity of illness variables, ICU medications administered and patient-centered outcomes of mortality and withdrawal of care. Multivariable models were constructed to assess statistical associations and risk factors for 7-d and 28-d mortality. Individual factors were included in the multivariable model if they were statistically significant to P < 0.10 in the univariate analysis, were clinically important, or have been shown in the literature to be of clinical significance. Univariate comparisons were performed using the Student-t test, Wilcoxon sign rank test, χ2 test, or Fisher exact test as appropriate. Multivariable models were constructed using Cox proportional hazards models and analysis of maximum likelihood estimates. Modeling both with composite MELD score and examining each variable in the MELD score independently were performed to ensure no one variable was dominant. Unadjusted, stratified Kaplan-Meier survival curves were constructed for 7-d and 28-d survival utilizing the log-rank test to determine statistical significance (P≤ 0.05). All statistical tests for significance were two-sided and a significance level p less than or equal to 0.05 was considered statistically significant. All data set manipulation and statistical analyses were performed using SAS (version 9.4, Cary, NC). Institutional review board approval was obtained for this study.
Forty-five consecutive patients with cirrhosis were treated for catecholamine-resistant septic shock; 21 received AVP as the second-line vasopressor while the remaining 24 received some other agent [phenylephrine (10), dopamine (6), norepinephrine (4), dobutamine (2), milrinone (2)]. Mean age was 57.2 ± 14.0 years. The cohort was 53.3% male and nearly ¾ had either alcoholic liver disease or chronic hepatitis C as the underlying etiology of cirrhosis (alcoholic alone 35.6%, chronic hepatitis C alone 26.7%, concomitant alcohol and hepatitis C 8.9%). All patients had either Child-Turcotte-Pugh Class B (n = 8, 14.5%) or Class C (n = 37, 85.5%) liver disease. Mean MELD score was 29.0 ± 9.0. Overall 7-d and 28-d mortality were 55.6% and 84.4% respectively, with two patients eventually undergoing liver transplantation at 34 and 67 d out from diagnosis of CRSS, respectively.
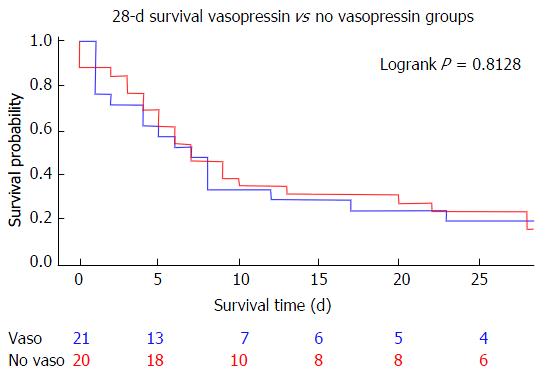
In general, no significant differences in baseline demographics, etiology of cirrhosis, laboratory values, vital signs or ICU mortality/severity of illness scores were observed when comparing those subjects who received AVP to those who received any other vasoactive agent, with the exception of higher MELD scores in the AVP group (32.4, 95%CI: 28.6-36.2 vs 27.1, 95%CI: 23.6-30.6, P = 0.041) (Table 1). Glomerular filtration rates were also different between the two groups (23.9 mL/min, 95%CI: 18.6-29.2 in the AVP group vs 40.0 mL/min, 95%CI: 29.1-51.0 in the non-AVP group, P = 0.013). Mean APACHE II scores were statistically similar (33.5, 95%CI: 30.6-36.5 in the AVP group vs 31.8, 95%CI: 29.4-34.2) as were SAPSII (72.6, 95%CI: 63.5-81.7 in the AVP group vs 70.3, 95%CI: 64.5-76.1 in the non-AVP group) and SOFA (17.6, 95%CI: 15.9-19.3 AVP vs 16.9, 95%CI: 15.9-18.0 non-AVP). Corticosteroid administration was also statistically similar (76.2% AVP vs 79.2% non-AVP) as was time to first vasopressor initiation (6.8, 95%CI: 4.9-8.7 h AVP vs 7.4, 95%CI: 5.7-9.3 h non-AVP). No statistically significant difference was observed in unadjusted 7-d mortality (52.4% AVP vs 58.3% and P = 0.408) or 28-d mortality (81.0% AVP vs 87.5% non-AVP, P = 0.813) (Figure 2). There was also no significant change in any recorded laboratory value of interest as measured 24-48 h after vasopressor initiation (Table 2).
| Vasopressin (n = 21) | No Vasopressin (n =24) | P value | |
| Patient demographics | |||
| Age, yr (95%CI) | 56.2 (50.2-62.3) | 57.0 (50.7-63.3) | 0.681 |
| Male gender | 10 (47.6) | 14 (53.9) | 0.672 |
| Body mass index, kg/m2, (95%CI) | 34.2 (30.5-37.9) | 31.2 (28.0-34.3) | 0.150 |
| Comorbidities, n (%) | |||
| CAD | 3 (14.2) | 4 (16.7) | 0.985 |
| CHF | 1 (5.3) | 6 (23.1) | 0.103 |
| COPD | 3 (16.7) | 4 (16.7) | 1.00 |
| CKD | 6 (28.6) | 7 (29.2) | 0.956 |
| DM | 7 (35.0) | 8 (30.8) | 0.762 |
| HTN | 13 (61.3) | 16 (66.7) | 0.916 |
| Smoking, n (%) | 5 (23.8) | 5 (23.8) | 0.756 |
| Alcohol use (active), n (%) | 9 (42.9) | 8 (33.3) | 0.392 |
| Liver disease etiology, n (%) | |||
| Alcohol | 6 (28.6) | 10 (41.7) | 0.477 |
| NASH/crypto | 5 (23.4) | 7 (29.2) | 0.240 |
| HBV | 0 (0.0) | 0 (0.0) | 1.00 |
| HCV | 3 (14.2) | 3 (12.5) | 0.566 |
| Cardiac | 1 (4.8) | 1 (4.2) | 0.947 |
| Cholestatic | 2 (9.5) | 1 (4.2) | 0.445 |
| AIH | 0 (0.0) | 1 (4.2) | 0.497 |
| HCV/alcohol | 3 (14.3) | 1 (4.2) | 0.329 |
| PSE | 14 (66.7) | 15 (62.5) | 0.927 |
| Laboratory values and vital signs | |||
| MELD, (95%CI) | 32.4 (28.6-36.2) | 27.1 (23.6-30.6) | 0.041 |
| CTP, n (%) | |||
| A | 0 (0.0) | 0 (0.0) | 1.00 |
| B | 2 (9.5) | 6 (25.0) | 0.074 |
| C | 19 (90.5) | 18 (75.0) | 0.162 |
| AST, U/L, (95%CI) | 429 (283-1141) | 289 (90-667) | 0.763 |
| ALT, U/L, (95%CI) | 180 (79-438) | 133 (24-290) | 0.795 |
| Alk phos, U/L, (95%CI) | 155 (109-200) | 138 (90-185) | 0.740 |
| Bilirubin, mg/dL, (95%CI) | 15.4 (9.0-21.9) | 10.0 (5.3-14.6) | 0.109 |
| BUN, mg/dL, (95%CI) | 58.0 (45.0-70.9) | 48.7 (36.5-60.9) | 0.222 |
| Platelets, k/uL, (95%CI) | 84.5 (66.2-102.8) | 88.8 (68.9-108.8) | 0.402 |
| Creatinine, mg/dL, (95%CI) | 3.02 (2.16-3.88) | 2.50 (1.59-3.41) | 0.37 |
| GFR, mL/min per 1.73 m2, (95%CI) | 23.9 (18.6-29.2) | 40.0 (29.1-51.0) | 0.013 |
| Sodium, mmol/L, (95%CI) | 135.8 (131.8-139.8) | 134.1 (130.8-137.5) | 0.553 |
| INR, (95%CI) | 2.63 (1.79-3.48) | 2.15 (1.82-2.47) | 0.176 |
| Hematocrit, %, (95%CI) | 25.7 (22.9-28.6) | 28.0 (26.1-30.0) | 0.200 |
| Lactate, mmol/L, (95%CI) | 3.90 (2.58-5.21) | 3.60 (2.52-4.68) | 0.669 |
| WBC (max), k/uL, (95%CI) | 16.1 (12.8-19.5) | 16.7 (12.7-20.6) | 0.607 |
| Heart rate, (95%CI) | 106 (96-115) | 110 (102-118) | 0.591 |
| MAP (min), (95%CI) | 45.1 (34.2-56.1) | 50.5 (46.9-54.0) | 0.197 |
| Temperature, C, (95%CI) | 36.3 (35.5-37.1) | 36.7 (35.9-37.4) | 0.125 |
| RR (max), breaths/min, (95%CI) | 35.7 (30.5-40.8) | 31.8 (25.4-38.3) | 0.145 |
| ICU level of illness, (95%CI) | |||
| FiO2 | 0.48 (0.36-0.59) | 0.44 (0.34-0.54) | 0.953 |
| PaCO2 | 35.6 (32.7-38.5) | 35.7 (32.1-39.3) | 0.856 |
| PaO2 | 100.2 (49.1-151.4) | 70.4 (60.2-80.6) | 0.235 |
| pH | 7.30 (7.24-7.35) | 7.34 (7.30-7.37) | 0.149 |
| APACHE II | 33.5 (30.6-36.5) | 31.8 (29.4-34.2) | 0.306 |
| GCS | 7.1 (5.0-9.3) | 6.9 (5.2-8.6) | 0.547 |
| SAPSII | 72.6 (63.5-81.7) | 70.3 (64.5-76.1) | 0.975 |
| SOFA | 17.6 (15.9-19.3) | 16.9 (15.9-18.0) | 0.173 |
| Average number of vasopressors | 2.9 (2.4-3.3) | 3.3 (2.9-3.6) | 0.357 |
| Days on vasopressors | 6.3 (3.7-8.9) | 6.3 (3.6-9.0) | 0.756 |
| CRRT/HD, n (%) | 13 (65.0) | 17 (70.8) | 0.762 |
| Intubated, n (%) | 18 (85.7) | 22 (91.7) | 0.466 |
| UOP first 24 h, mL, (95%CI) | 459.9 (225.8-694.0) | 698.1 (383.9-1012.3) | 0.067 |
| GI bleed, n (%) | 1 (20.0) | 5 (20.8) | 0.948 |
| New VTE, n (%) | 4 (20.0) | 3 (12.5) | 0.635 |
| ICU medications | |||
| Volume of IVF (L), (95%CI) | 4.02 (2.52-5.53) | 4.44 (2.62-6.26) | 0.891 |
| Octreotide, n (%) | 14 (66.7) | 12 (52.2) | 0.329 |
| Antibiotics, n (%) | 21 (100.0) | 24 (100.0) | 0.790 |
| Choice of first vasopressor, n (%) | |||
| Norepinephrine | 18 (85.7) | 17 (70.8) | 0.412 |
| Dopamine | 1 (4.8) | 3 (12.5) | 0.398 |
| Phenylephrine | 2 (9.5) | 4 (16.7) | 0.207 |
| Albumin given, n (%) | 18 (85.7) | 21 (95.5) | 0.954 |
| PPI, n (%) | 18 (90.0) | 19 (79.2) | 0.388 |
| Corticosteroids, n (%) | 16 (76.2) | 19 (79.2) | 0.701 |
| Outcomes, (95%CI) | |||
| Days to death | 8.9 (5.2-11.4) | 7.8 (4.4-11.1) | 0.672 |
| ICU LOS, d | 13.5 (8.1-18.8) | 12.3 (4.4-20.3) | 0.114 |
| Vent free days | 22.6 (20.1-25.1) | 15.8 (4.1-27.6) | 0.633 |
| Mortality, n (%) | |||
| 7 d | 11 (52.4) | 14 (58.3) | 0.408 |
| 28 d | 17 (81.0) | 21 (87.5) | 0.371 |
| 90 d | 18 (85.7) | 21 (87.5) | 0.303 |
| In hospital | 18 (85.7) | 20 (83.3) | 0.654 |
| ICU | 17 (81.0) | 18 (75.0) | 0.360 |
| Transition to comfort care | 16 (76.2) | 18 (75.0) | 0.808 |
| Vasopressin (n = 21) | No Vasopressin (n = 24) | P value | |
| Platelets, k/uL, (95%CI) | -18.7 (-42.3, 4.9) | -13.6 (-31.6, 4.4) | NS |
| ALT, U/L, (95%CI) | 47.2 (-12.1, 106.6) | 206.3 (-113.3, 525.9) | NS |
| AST, U/L (95%CI) | 236.7 (74.0, 399.4) | 292.4 (-247.0, 831.8) | NS |
| Alkaline phosphatase, U/L, (95%CI) | -10.5 (-48.7, 27.8) | -19.6 (-39.5, 0.3) | NS |
| Heart rate, (95%CI) | -6.7 (-12.3, -1.0) | 0.6 (-11.8, 13.0) | NS |
| Bilirubin, mg/dL, (95%CI) | 0.45 (-0.99, 1.89) | 0.87 (-0.64, 2.38) | NS |
On adjusted multivariable analysis, AVP use was not associated with increased 28-d mortality (HR = 0.77, 95%CI: 0.39-1.52, P = 0.771). Age in years (HR = 1.05, 95%CI: 1.01-1.08, P = 0.004) was associated with increased 28-d mortality (Table 3). In other words, for each addition year of age from the baseline cohort average, the mortality rate was increased 5%. Corticosteroid administration was a significant predictor of improved 28-d mortality (HR = 0.37, 95%CI: 0.16-0.86, P = 0.021). The initiation of renal replacement therapy was associated with lower mortality (HR = 0.40, 95%CI: 0.19-0.85, P = 0.017). No significant difference was found for MELD score.
After adjusting for multiple confounding factors, we report that AVP is not associated with disparate outcomes when compared to all other vasoactive agents in terms of 7-d and 28-d mortality when used as a second line vasopressor in catecholamine-resistant septic shock. These results are particularly notable considering the extent to which our AVP group was comprised of patients with a higher severity of illness as reflected by statistically higher baseline MELD scores as well as severity of illness scores which, while not individually differing statistically between the two groups, nevertheless all tended to be higher in the AVP group. Estimated glomerular filtration rates were also significantly lower in the AVP group, however these data need to be interpreted with caution as several of these patients were already receiving some form of renal replacement therapy at the time of vasopressor initiation. Additionally, we report no statistically significant difference in the total number of vasoactive agents used among the groups with both groups receiving approximately three such agents during the study period, a surrogate outcome which may indicate that AVP did not impair attainment of target mean-arterial pressures when compared with other agents. We do acknowledge that, due to the high rate of transition to comfort care measures, these data should also be interpreted cautiously, nevertheless rates of changes in goals of care were essentially equivalent in the 2 groups. Well-designed, prospective, randomized studies are needed to clarify whether AVP should be preferred as the second-line vasopressor in this patient population.
Potential adverse effects of AVP administration were not different when compared to all other vasoactive agents. While others have published reports suggesting acute-on-chronic liver failure, worsening thrombocytopenia and a decline in cardiac output with AVP use[4,12-17] our results do not lend support to these concerns during early treatment, as we did not find any significant laboratory changes in these parameter between the two groups as measured 24-48 h after vasopressor initiation. Consonant with these findings, we report similar rates of de novo venous thromboembolic disease among the two groups. While direct measurement of cardiac output or cardiac index was not obtainable in our retrospective analysis, heart rate did not decline significantly after one-day of vasopressor therapy in the AVP group when compared with the non-AVP group, lessening concerns regarding clinically significant negative chronotropy affecting cardiac output in this population. Although some reports suggest mortality benefit with attenuation of tachycardia in patients with septic shock, a decline in cardiac output mediated by decreased heart rate may have a disparate and adverse effect in cirrhosis patients when compared to the general population given the possible underlying dependence of oxygen consumption on oxygen delivery in this population[18,20].
From a safety and efficacy standpoint, our findings confirm a salient role for AVP use in cirrhosis patients with CRSS and strengthen the current level of evidence provided in support of recent consensus guidelines for critical care in patients with cirrhosis which are based largely on data extrapolated from studies of terlipressin administration[11].
On adjusted multivariable analysis, corticosteroid use emerged as a marked predictor of improved 28-d mortality with a 63% reduction in death with corticosteroid administration. Current Surviving Sepsis guidelines do recommend low-dose hydrocortisone for patients with septic shock unresponsive to fluid resuscitation and 60 min of vasopressors support. However, while the prevalence of adrenal insufficiency among patients with cirrhosis and sepsis has been generally reported as higher than expected, upwards of some 76% of this population, a recent randomized-controlled trial did not evidence a mortality benefit when stress-dosed steroids were employed in the ICU management of these patients[10]. In a randomized, placebo-controlled trial of 75 cirrhosis patients admitted to an intensive care unit with septic shock that was stopped early due to futility, Arabi et al[10] reported a 28-d mortality of 85% in the group of patients randomized to receive low-dose corticosteroids compared with 72% in the placebo-allocated group. While our mortality rates approximate those in the steroid-receiving group reported by Russell et al[21] it is clear that our patients suffering CRSS represented a more critically ill population as evidenced not only by a pre-specified requirement for 2 or more vasopressors, but also by the higher APACHE II and SOFA scores which characterized our patients. While the discrepancy regarding steroid-benefit may be real and attributable to the differing populations under study, another intriguing hypothesis which emerged from a post-hoc substudy of VASST relates to a possible beneficial synergy between AVP and corticosteroid, with the authors of this substudy reporting a decrease in 28-d mortality from 44.7% to 35.9% in patients receiving corticosteroids plus AVP when compared with patients receiving corticosteroids in addition to norepinephrine.
Finally, rates of gastrointestinal hemorrhage, including that from gastroesophageal varices, were also similar between the AVP and non-AVP groups.
Our study has several limitations. First, it is retrospective in nature and suffers from missing data, a deficiency common to most retrospective analyses. Second, ours is a single center study with a relatively small sample size constraining analysis of additional variables. Third, we acknowledge the heterogeneity of the comparative group regarding the variety of second-line agents used. However, on the other hand, a salient feature of this study is that the 2nd vasoactive agent used in the comparator group was almost exclusively a catecholaminergic agent, which in effect resulted in a study comparing second-line vasopressin use vs second-line catecholaminergic augmentation.
Fourthly, an additional limitation relates to “cross-over” analysis, as we did not analyze our cohort of patients on the basis of whether or not they received AVP at any time during their course. Furthermore, we did not investigate the possible interaction between AVP and corticosteroids as discussed earlier. Our study is also relatively underpowered given the high 28-d mortality rates observed and the low-even rate of patient survival. Other limitations include a lack of direct measurement of cardiac output or index with right heart catheterization in order to better characterize changes in hemodynamics following AVP administration.
Nevertheless, we provide more methodologically robust evidence for AVP use as a second-line vasopressor in catecholamine resistant septic shock and for attention to vasopressor selection in patients with cirrhosis. While further, large-scale multicenter prospective studies would be of benefit to refine current consensus standards, all potential lifesaving interventions, as long as the potential for iatrogenic harm is minimal, should be considered in this extremely sick patient population with 28-d mortality rates approaching 85%. Ultimately, the goal of correcting catecholamine-resistant septic shock in these patients involves both recovery from their immediate, life-threatening illness as well as providing for relative convalescence which may enable the individual patient to recover and receive a liver transplantation.
Cirrhosis patients with septic-shock requiring intensive care unit medical care have an exceedingly high mortality rate and are excluded from many existing clinical trials. Recent consensus guidelines suggest a role for vasopressin use in this patient population; however, this is based largely on expert opinion.
With the increasing prevalence of cirrhosis globally and improved access to tertiary medical care, the care of the critically ill patient with cirrhosis of the liver cannot be ignored. Current research and clinical care focuses largely on keeping the critically ill patient with cirrhosis alive in order to eventually receive a life-saving liver transplantation. The role of vasopressin in this population remains unknown.
In the present study, the authors found that vasopressin is similar to all other vasopressors in terms of 7-d and 28-d mortality and in the absence of significantly more deleterious effects suggest a role for vasopressin use in patients with cirrhosis admitted to the intensive care unit with septic shock.
The present report provides further evidence on the safety and efficacy of vasopressin use in patients with cirrhosis, and may suggest revisiting the currently available critical care guidelines.
This retrospective cohort adds useful information for both clinical practice and further academic research with the goal of impacting common patient centered outcomes for critically ill patients with extremely high mortality rates.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Giorgio A, Tellez-Avila F, Zhu YY S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Pavon A, Binquet C, Kara F, Martinet O, Ganster F, Navellou JC, Castelain V, Barraud D, Cousson J, Louis G. Profile of the risk of death after septic shock in the present era: an epidemiologic study. Crit Care Med. 2013;41:2600-2609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Galbois A, Aegerter P, Martel-Samb P, Housset C, Thabut D, Offenstadt G, Ait-Oufella H, Maury E, Guidet B. Improved prognosis of septic shock in patients with cirrhosis: a multicenter study. Crit Care Med. 2014;42:1666-1675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Moreau R, Hadengue A, Soupison T, Kirstetter P, Mamzer MF, Vanjak D, Vauquelin P, Assous M, Sicot C. Septic shock in patients with cirrhosis: hemodynamic and metabolic characteristics and intensive care unit outcome. Crit Care Med. 1992;20:746-750. [PubMed] [Cited in This Article: ] |
| 4. | Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1191] [Cited by in F6Publishing: 1128] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 5. | Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122-1125. [PubMed] [Cited in This Article: ] |
| 6. | Landry DW, Levin HR, Gallant EM, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997;25:1279-1282. [PubMed] [Cited in This Article: ] |
| 7. | Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D. Circulating vasopressin levels in septic shock. Crit Care Med. 2003;31:1752-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Wagener G, Kovalevskaya G, Minhaz M, Mattis F, Emond JC, Landry DW. Vasopressin deficiency and vasodilatory state in end-stage liver disease. J Cardiothorac Vasc Anesth. 2011;25:665-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Fernández J, Escorsell A, Zabalza M, Felipe V, Navasa M, Mas A, Lacy AM, Ginès P, Arroyo V. Adrenal insufficiency in patients with cirrhosis and septic shock: Effect of treatment with hydrocortisone on survival. Hepatology. 2006;44:1288-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Knawy BA, Hajeer AH, Tamimi W, Cherfan A. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182:1971-1977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Nadim MK, Durand F, Kellum JA, Levitsky J, O’Leary JG, Karvellas CJ, Bajaj JS, Davenport A, Jalan R, Angeli P. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J Hepatol. 2016;64:717-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 191] [Article Influence: 23.9] [Reference Citation Analysis (1)] |
| 12. | Leone M, Albanèse J, Delmas A, Chaabane W, Garnier F, Martin C. Terlipressin in catecholamine-resistant septic shock patients. Shock. 2004;22:314-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Morelli A, Ertmer C, Lange M, Westphal M. Continuous terlipressin infusion in patients with septic shock: less may be best, and the earlier the better? Intensive Care Med. 2007;33:1669-1670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Albanese J, Leone M, Delmas A, Martin C. Terlipressin or norepinephrine in hyperdynamic septic shock: A prospective, randomized study. Crit Care Med. 2005;33:1897-1902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | O’Brien A, Clapp L, Singer M. Terlipressin for norepinephrine-resistant septic shock. Lancet. 2002;359:1209-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Luckner G, Dünser MW, Jochberger S, Mayr VD, Wenzel V, Ulmer H, Schmid S, Knotzer H, Pajk W, Hasibeder W. Arginine vasopressin in 316 patients with advanced vasodilatory shock. Crit Care Med. 2005;33:2659-2666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Umgelter A, Reindl W, Schmid RM, Huber W. Continuous terlipressin infusion in patients with persistent septic shock and cirrhosis of the liver. Intensive Care Med. 2008;34:390-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Moreau R, Lee SS, Hadengue A, Ozier Y, Sicot C, Lebrec D. Relationship between oxygen transport and oxygen uptake in patients with cirrhosis: effects of vasoactive drugs. Hepatology. 1989;9:427-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3541] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 20. | Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, Orecchioni A, D’Egidio A, D’Ippoliti F, Raffone C. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683-1691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 490] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 21. | Russell JA, Walley KR, Gordon AC, Cooper DJ, Hébert PC, Singer J, Holmes CL, Mehta S, Granton JT, Storms MM. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Crit Care Med. 2009;37:811-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |