Published online Nov 18, 2016. doi: 10.4254/wjh.v8.i32.1384
Peer-review started: June 28, 2016
First decision: September 5, 2016
Revised: September 23, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: November 18, 2016
Processing time: 141 Days and 12.5 Hours
To assess the value of the mean systemic-to-pulmonary artery pressure (MAP/mPAP) ratio for predicting outcomes following orthotopic liver transplant (OLT).
A retrospective data analysis was performed and data (mean arterial blood pressure, mean pulmonary artery pressure and Cardiac Index) were collected at several points during OLT. Outcomes evaluated were duration of postoperative endotracheal intubation [ET; minutes after intensive care unit (ICU) arrival], length of ICU stay, total hospitalization and frequency of immediate postoperative complications. A total of 91 patients were included in the data analysis. Based on the intraoperative course of the MAP/mPAP ratio, 2 hemodynamic responses were identified: Group 1 (MAP/mPAP ratio increase during anhepatic period with postreperfusion recovery, n = 66); and Group 2 (MAP/mPAP ratio with no change during anhepatic period or decreased without recovery, n = 25).
The main finding was that the lack of increased MAP/mPAP ratio in the anhepatic period was associated with: (1) longer intubation times; and (2) prolonged ICU stays and total hospitalization time, when compared to patients with an increase in MAP/mPAP ratio during the anhepatic period.
The data from this retrospective study should raise awareness to the mean systemic to pulmonary artery pressure ratio as a potential indicator for poor outcome after OLT. Further prospective studies are needed for validation.
Core tip: The aim of this study was to assess the value of the mean systemic-to-pulmonary artery pressure (MAP/mPAP) ratio for predicting outcomes following orthotopic liver transplant. The intraoperative pattern of this ratio has not been previously described. Performing a retrospective analysis we identified 2 different MAP/mPAP patterns: Group 1 (MAP/mPAP ratio increase during anhepatic period with postreperfusion recovery, n = 66); and Group 2 (MAP/mPAP ratio with no change during anhepatic period or decreased without recovery, n = 25). The main finding was that the lack of increased MAP/mPAP ratio in the anhepatic period was associated with longer intubation times, and prolonged hospitalization time.
- Citation: Rebel A, Nguyen D, Bauer B, Sloan PA, DiLorenzo A, Hassan ZU. Systemic-to-pulmonary artery pressure ratio as a predictor of patient outcome following liver transplantation. World J Hepatol 2016; 8(32): 1384-1391
- URL: https://www.wjgnet.com/1948-5182/full/v8/i32/1384.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i32.1384
The mean systemic-to-pulmonary artery pressure ratio (MAP/mPAP) has been shown to be a valuable predictor of outcomes following cardiac surgery. Previous studies documented that the MAP/mPAP ratio was easy to obtain during cardiac surgery, and correlated with the development of pulmonary hypertension and diastolic dysfunction[1-4]. Outcomes following orthotopic liver transplant (OLT) are dependent on the ability of the patient’s cardiovascular system to compensate for the physiological stress related to OLT. While detailed and expensive cardiac evaluation is routinely performed on patients before OLT, the extent of cirrhotic cardiomyopathy and biventricular dysfunction is often underestimated[5,6]. Intraoperatively, due to circumstances related to advanced multi-organ disease, limited cardiac reserve and procedural related stressors including blood loss, fluid shifts, acidosis, or hypothermia, the patient may present more hemodynamic challenges than anticipated[7].
The MAP/mPAP ratio as a predictor of patient outcome following OLT has not been investigated. We hypothesized that the pattern of the MAP/mPAP ratio during the different stages of OLT may predict the ability of the circulatory system to compensate for the surgery related stress. If the MAP/mPAP pattern indicates sufficient cardiac reserve, the patient should have a better outcome than patients with a MAP/mPAP ratio that is less favorable. In order to more reliably risk stratify these patients undergoing OLT, we performed a retrospective data analysis to explore the feasibility of obtaining useable data during OLT. With desirable outcomes defined as less morbidity/mortality, decreased need for mechanical ventilation and shorter length of stay, the aim of this study was to assess the value of the MAP/mPAP ratio for predicting desirable outcomes following OLT.
The Institutional Review Board (IRB) reviewed the study protocol and gave approval access to an institutional database to retrieve patient information. The IRB waived the need for informed consent since the de-identified data review demonstrated minimal risk to patient population.
The retrospective data analysis was performed on patients undergoing OLT for end-stage liver disease in the time period from October 2011 through October 2014 at a single University Hospital. All patients undergoing OLT during this time period regardless of underlying liver disease, model for end-stage liver disease (MELD) score and age were included. Exclusion criteria were patients undergoing OLT as a combined procedure with kidney transplant, redo-OLT or OLT as a treatment for acute liver failure. Patient records with incomplete intraoperative information were excluded from the data analysis.
The selected time period was based on the absence of changes in surgical approach or staff (transplant surgeon/anesthesiology). All patients underwent cardiac evaluation with transthoracic echocardiography prior to listing for liver transplant. None of the included patients were diagnosed with or had signs of pulmonary hypertension prior to OLT.
All OLT were performed using the end-to-end inferior vena cava (IVC) anastomosis technique requiring total IVC cross-clamp during the anhepatic period. Using this technique, the anhepatic period was less than 60 min for all OLTs in this study period. Intraoperative anesthesia care for all patients followed a standardized protocol for anesthesia induction, intravenous access, monitoring and vasoactive medications. All patients remained intubated at the conclusion of their surgical procedure and were transported to a dedicated intensive care unit (ICU) for anesthesia emergence and recovery.
Patient demographic data were collected including preoperative creatinine level, comorbidities, MELD score, age and gender. Basic intraoperative information such as procedure time, intraoperative intravenous fluids and blood component therapy were extracted from the surgical records. During the retrospective chart review, the following intraoperative hemodynamic data were gathered: Mean arterial blood pressure, mean pulmonary artery pressure and Cardiac Index (CI). These hemodynamic parameters were collected at several time points during the surgical procedure: Baseline (30 min after incision), pre-anhepatic (1 h before IVC cross-clamp), anhepatic (15 min before reperfusion), neo-hepatic (15 min after reperfusion), and 1 h neo-hepatic (1 h after reperfusion).
Based on pilot observations, the MAP/mPAP ratio was expected to increase during the anhepatic phase. During the data analysis, patients indicating an increase in MAP/mPAP ratio by ≥ 1 from baseline to anhepatic phase were categorized into Group 1. Patients showing a decrease in MAP/mPAP ratio of ≥ -1 or no change (< 1 to > -1) from baseline to anhepatic phase were categorized into Group 2. We chose the anhepatic period as comparison to the baseline value because this surgical stage is characterized by a single hemodynamic alteration (preload reduction) and for a prolonged duration. In our institution, the anhepatic period is approximately 45-55 min. Therefore, all patients received a similar type of cardiac stress. To account for fluctuations in this anhepatic period we chose a measurement point at 15 min before reperfusion (IVC cross-clamp release) to allow sufficient equilibration time for reduction in cardiac preload caused by the IVC flow interruption.
Further chart review was performed regarding the use of vasoactive agents in the anhepatic phase of the operation. The following medications are available intraoperatively: Norepinephrine (NE), Epinephrine (EPI), Vasopressin (V), Dopamine (DOP) and Phenylephrine (PHE). Intraoperative documentation allowed the investigators to identify of the frequency and dosing of vasoactive medication at 30 to 15 min before reperfusion (anhepatic measurement of MAP/mPAP ratio). The patients were sorted into three categories: No vasoactive medication use, low dose vasoactive medication use (NE < 0.05 μ/kg per minute, EPI < 0.03 μg/kg per minute, V < 0.03 units/min, or PHE < 0.1 μg/kg per minute) and high dose vasoactive medication use (NE ≥ 0.05 μg/kg per minute, EPI ≥ 0.03 μg/kg per minute, V ≥ 0.03 units/min or any vasopressor combination).
The patient outcomes evaluated were duration of postoperative endotracheal intubation (ET, minutes after ICU arrival), length of ICU stay (LOS ICU, days post OLT) and length of hospital stay (LOS Total, days post OLT to hospital discharge). Postoperative complications were recorded if they occurred in the first 14 postoperative days after OLT. The frequency of reintubation within 48 h post extubation, need for renal replacement therapy and the need for ICU readmission were recorded. Mortality < 1 mo post OLT was also recorded.
Data is reported as mean ± SD. Statistical analysis was performed by the paired t-test or χ2 test. A P value of < 0.05 was used to identify statistical significance.
A total of 100 patients were included in the study. Due to incomplete data recordings, 9 patients were excluded from data analysis. Thus, data from a total of 91 patients was included in this analysis. The demographic patient characteristics and characterization of intraoperative course are shown in Table 1. Age, gender and MELD scores were equally distributed in both groups. The most common causes for end-stage liver disease in our patient collective were Hepatitis C related liver cirrhosis (48 patients, Group 1: 35 patients; Group 2: 13 patients), NASH related cirrhosis (21 patients, Group 1: 16 patients; Group 2: 5 patients) and alcohol induced liver cirrhosis (25 patients, Group 1: 16 patients; Group 2: 9 patients). Primary sclerosing cholangitis related liver cirrhosis was the leading diagnosis in 5 patients (all in Group 1). Other rare OLT indications were autoimmune hepatitis or alpha-trypsin 2 deficiency (one patient each, all Group 1). Two patients had hepato-pulmonary syndrome prior to OLT (both in Group 1).
| Age (yr) | Gender (F:M ratio) | MELD (score) | OR duration (min) | Crystalloid (mL) | Colloid (mL) | PRBC (units) | FFP (units) | |
| Group 1 (n = 66) | 55.6 ± 8.6 | 24:42 (36%) | 18.7 ± 8.4 | 400.9 ± 43.5 | 5804 ± 2824 | 1440 ± 972 | 3.3 ± 3.7 | 2.8 ± 3.0 |
| Group 2 (n = 25) | 58.9 ± 5.4 | 7:18 (28%) | 16.3 ± 6.9 | 427.9 ± 63.3 | 6086 ± 3424 | 1607 ± 626 | 3.9 ± 3.1 | 4.0 ± 4.6 |
| P-value | 0.081 | 0.189 | 0.204 | 0.081 | 0.690 | 0.428 | 0.473 | 0.148 |
Pre-OLT Creatinine was not different between the two groups (Group 1: 1.26 ± 0.67 mg/dL, Group 2: 1.30 ± 0.82 mg/dL). There were no significant differences in surgical duration, fluid requirements or use of blood component therapy between the patient collectives (Table 1).
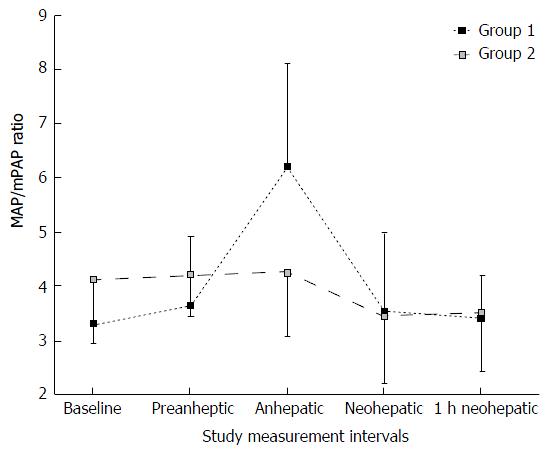
The intraoperative MAP/mPAP values for each group at the different measurement points are shown in Figure 1. MAP/mPAP values at baseline and at the preanhepatic period were slightly higher in Group 2 than in Group 1 (Table 2). However, the difference was not statistically significant. Patients in Group 1 demonstrated a significant increase in MAP/mPAP during the anhepatic period. The MAP/mPAP ratio pattern in Group 2 showed less variability throughout the surgical procedure (Figure 1). The analysis of the absolute MAP/mPAP values indicated that MAP/mPAP recovered to baseline range after reperfusion in both groups. However, the percent change of MAP/mPAP ratio at baseline to the different measurement points indicated that MAP/mPAP recovered to baseline after reperfusion in Group 1. The percent change of MAP/mPAP ratio was significantly decreased in Group 2 after reperfusion, lasting up to 1 h post reperfusion (Table 2). In Group 1 the MAP/mPAP ratio increased by 2.9 ± 1.8 during the anhepatic period, while MAP/mPAP ratio in Group 2 only changed by 0.1 ± 1.1 at this observation point. CI measurements did not parallel the hemodynamic patterns shown by the MAP/mPAP ratio (Table 2). There were no significant changes in CI between both groups at any observation points during the surgical procedure.
| Group 1 | Group 2 | P-value | |
| MAP/mPAP increase during anhepatic period (n = 66) | MAP/mPAP no change or decrease during anhepatic period (n= 25) | ||
| MAP/mPAP baseline | 3.32 ± 0.734 | 4.13 ± 1.191 | 0.185 |
| % change preanhepatic | 0.278 ± 1.276 | 0.184 ± 1.268 | 0.754 |
| % change anhepatic | 2.892 ± 1.827 | -0.088 ± 1.137 | < 0.01a |
| % change neohepatic | 0.206 ± 1.411 | -0.688 ± 1.325 | < 0.01a |
| % change 1 h neohepatic | 0.098 ± 0.828 | -0.611 ± 1.373 | < 0.01a |
| CI baseline | 4.111 ± 1.229 | 4.214 ± 0.961 | 0.879 |
| % change preanhepatic | 0.039 ± 0.790 | -0.204 ± 0.832 | 0.200 |
| % change anhepatic | -1.606 ± 1.018 | -1.188 ± 1.082 | 0.089 |
| % change neohepatic | -0.327 ± 1.316 | -0.058 ± 1.231 | 0.378 |
| % change 1 h neohepatic | 0.335 ± 1.106 | 0.454 ± 1.038 | 0.643 |
| ET | |||
| min | 967 ± 1361 | 1719 ± 1933 | 0.040a |
| h | 16.1 ± 22.7 | 28.7 ± 32.2 | |
| Median (min) | 540 | 1045 | |
| LOS ICU (d) | 3.9 ± 4.4 | 12.1 ± 19.2 | < 0.01a |
| Median (d) | 2 | 6 | |
| LOS hospital (d) | 12.0 ± 12.5 | 26.3 ± 33.2 | < 0.01a |
| Median (d) | 8 | 11 |
The majority of patients required vasoactive medication during the anhepatic phase and the presence of these medications may have influenced the hemodynamic measurements at the anhepatic measurement point (Table 3). Approximately 80% of patients in both groups received some vasopressor assistance during the anhepatic phase. Phenylephrine, Vasopressin and Norepinephrine were the most commonly used vasopressors. While approximately half of patients required some low-dose vasoactive medication, the frequency and severity of vasopressor support was not different between Group 1 and 2.
| Group 1 | Group 2 | P-value | |
| Vasopressor use frequency | MAP/mPAP increase during anhepatic period (n = 66) | MAP/mPAP no change or decrease during anhepatic period (n = 25) | |
| No vasopressor use | 14 | 5 | 0.624 |
| Low dose vasopressor use | 39 | 12 | 0.341 |
| High dose vasopressor use | 13 | 8 | 0.214 |
There were significant differences in duration of postoperative intubation, length of stay in the ICU and length of hospital stay between Group 1 and Group 2 (Table 2). Patients in Group 1 extubated on an average of 12 h earlier than the patients in Group 2 (16.1 ± 22.7 h vs 28.7 ± 32.2 h, respectively; P = 0.04). The duration of ICU stay was reduced by almost 8 d (Group 1: 3.9 ± 4.4 d, Group 2: 12.1 ± 19.2; P < 0.01). Total length of hospitalization was approximately 2 wk less in Group 1 than in Group 2 (12.0 ± 12.5 d vs 26.3 ± 33.2 d, respectively; P < 0.01).
In the immediate post OLT period (up to 30 d postoperatively) no mortality was reported in either group. In Group 1 four out of 66 patients (6%) required reintubation and five out of 66 patients (8%) received RRT; no other complications were reported in this group. In Group 2 three out of 25 patients (12%) required reintubation and seven out of 25 patients (28%) received RRT post OLT. One patient was readmitted to ICU and one patient developed seizures.
The main finding of this study was that despite having similar preoperative pathophysiology, one quarter of patients undergoing OLT did not display the expected increase of MAP/mPAP ratio during the anhepatic phase. This lack of increased MAP/mPAP ratio in the anhepatic period by > 1 compared to baseline values was associated with: (1) longer duration of postoperative intubation; and (2) prolonged ICU stay and total hospitalization time when compared to patients with an increase in MAP/mPAP ratio during the anhepatic period. The changes in MAP/mPAP ratio were not mirrored by CI, thus the intraoperative pattern of MAP/mPAP may be predictive of patient clinical outcomes after liver transplantation. To our knowledge, this is the first study to describe the intraoperative pattern of MAP/mPAP ratio during liver transplant and its possible relationship with patient outcomes following OLT.
Advanced liver disease has been known to affect other organ functions, most importantly the cardiovascular and renal systems[8,9]. The connection between liver cirrhosis and cardiac dysfunction has been previously recognized[8,10-12] as recent studies have documented that cirrhosis is associated with biventricular systolic dysfunction[5,6]. It is standard practice for patients to undergo an extensive preoperative cardiac evaluation to risk stratify them prior to listing for liver transplantation. The echocardiographic assessment and stress testing is usually centered on left ventricular function. While right ventricular systolic pressure and or Tricuspid annular plane systolic excursion is usually measured to evaluate the patient for right heart function and pulmonary hypertension; neither of these parameters correlates well with right systolic function[5]. Advanced echocardiography techniques (strain analysis/right ventricle relative area change) are rarely included in the standard pre OLT echocardiography assessment[5,13].
Cardiologists commonly use the MAP/mPAP ratio to stratify the severity of pulmonary hypertension because it describes the close relationship between systemic and pulmonary circulations[14]. Preoperative value of MAP/mPAP < 4 would be of concern for cardiologists for possible pulmonary hypertension, and MAP/mPAP values < 4 have been correlated with lower survival rates after cardiac surgery[2,15]. We used the MAP/mPAP ratio in this study to assess its value to predict outcomes after liver transplantation, since this parameter has shown to be a useful predictor of hemodynamic complications after cardiac surgery[2]. Our data confirm the previous findings of the use of MAP/mPAP to identify patients at risk for adverse events after high-risk surgery. In this study, the intraoperative hemodynamic MAP/mPAP pattern of patients undergoing OLT indicated that an increase in MAP/mPAP during the anhepatic phase is associated with better outcomes. A novelty of this report is the analysis of the MAP/mPAP ratio intraoperative pattern and correlating it with postoperative outcomes.
While our reported data are truly observational, it is not well established what significance the MAP/mPAP ratio represents, especially in patients with advanced liver disease. Cardiologists have set a normal value of > 4[14]. The observation that the majority of the included patients in our study had values < 4 in absence of pulmonary hypertension may be due to advanced liver disease or the effect of anesthetic medications, since the baseline measurements were taken post induction of anesthesia. Previous studies indicated only minor effect of anesthesia on the MAP/mPAP ratio[2]. However, patients with advanced liver disease may have lower numbers due to systemic vasodilation and may have an exaggerated vascular response to anesthetics.
A recent study from Bushyhead et al[16] investigated preoperative data of liver transplant recipients and found that the pulmonary artery systolic pressure correlates with posttransplant outcome and therefore emphasized the importance of right ventricular assessment and pulmonary vascular resistance for the morbidity and mortality associated with the procedure. However, the publication did not assess the value of the MAP/mPAP ratio for preoperative risk stratification. While our study did not obtain preoperative MAP/mPAP values prior to anesthesia induction and did not include patients with pre-existing pulmonary hypertension defined by elevated pulmonary artery pressures, the findings of our study support the need for more thorough assessment of right heart function and cardiac reserve prior to liver transplantation. With the scarcity of acceptable donor organs, the best surgical candidate with the least likelihood for postoperative complication needs to be identified. Including MAP/mPAP ratio into the preoperative assessment may provide useful information.
Minimizing the importance of a single measurement and focusing on the intraoperative patterns of the MAP/mPAP ratio, the trend of the parameter may be interpreted as an indication of contractility reserve. The hemodynamic hallmark of the anhepatic phase during liver transplantation is characterized by significant reduction in IVC flow and therefore blood return to the heart. The behavior of MAP/mPAP ratio during the anhepatic phase therefore may indicate the systemic and pulmonary circulatory response during reduced cardiac preload. An increase in MAP/mPAP ratio may suggest that circulatory systems are able to adjust to stress and hypovolemia by vasoconstriction and inotropic compensation. Therefore, the ability to increase the MAP/mPAP ratio in the anhepatic phase observed in Group 1 indicates better cardiovascular reserve than the lack of increase or decrease as observed in Group 2.
We chose to use the MAP/mPAP ratio difference between baseline and anhepatic phase because the anhepatic stage primarily represents a single hemodynamic alteration (preload reduction). Therefore, all patients received a similar type of cardiac stress and the MAP/mPAP ratio may be more representative for the cardiac ability to compensate for the preload reduction. Although hepatic reperfusion can cause significant cardiac strain, the cardiac response to reperfusion depends on multiple factors and some of them may be due to the donor organ. The duration of reperfusion is usually short and therefore changes in MAP/mPAP may be not reflecting the cardiac response to the changes in preload, cardiac contractility or afterload. Our study used single MAP/mPAP ratio measurements at predetermined measurement point representing the hemodynamic situation during the surgical stage. Due to the fluctuating nature of all hemodynamic parameters during OLT, a continuous assessment of MAP/mPAP ratio throughout the entire surgical procedure may be more desirable in future studies to describe the cardiac reserve.
In our study, vasoactive medications were not controlled during the surgical procedure, and were titrated to effect by the anesthesia provider to ensure hemodynamic stability. Variable doses of vasoactive medications were given in both study groups. However, drug selection and dosing did not appear to influence the MAP/mPAP pattern since there were no statistical differences in distribution between both groups.
In previous studies on cardiac patients without pre-existing pulmonary hypertension, a low MAP/mPAP ratio was found to be an independent predictor of difficult separation of cardiopulmonary bypass and right heart failure[2,17]. Robitaille et al[2] found that patients with lower MAP/mPAP ratios had more hemodynamic complications after cardiac surgery defined as cardiac arrest, vasopressor therapy > 24 h postop, and/or use of intra-aortic balloon pump postop. These findings are in agreement with our interpretation of MAP/mPAP ratio as a predictor of the ability of the cardiovascular system to provide hemodynamic compensation. If a MAP/mPAP increase during the anhepatic phase is interpreted as a positive cardiovascular response to stressors, the lack of such compensation would explain why the patients without such a response would have less favorable outcomes.
Robitaille et al[2] correlated the preoperative MAP/mPAP ratio with surgical outcome after cardiac surgery and reported that the preoperative MAP/mPAP ratio was significantly higher in survivors (3.9 ± 1.4) than in those who died (3.2 ± 1.4). Since the surgical procedure during liver transplantation has more complexity and varying hemodynamic challenges specific to each surgical phase, we chose (per expert consensus) observation points to describe the MAP/mPAP pattern throughout different surgical stages of the procedure. Our pattern analysis confirms the findings of the single preoperative measurement in the previous study[2]. However, our observation and current understanding of the ratio is that it is not a static parameter and, per our data, large ratio fluctuations can occur and should alert the clinician to initiate an adjustment in the treatment plan.
Our study has several limitations. First, all data was gathered as a retrospective study from only one institution without randomization or blinding. The normal hemodynamic response was defined by the authors based on preliminary observations and understanding of hemodynamic response to the IVC flow alterations during the anhepatic phase. However, this categorization may be oversimplified to demonstrate the variety of possible dynamic responses in the anhepatic period. The study was also limited by data analysis. The data was not measured continuously, as we selected the ratio of mean systemic to pulmonary artery pressure at defined time points during the liver transplant. Our endpoint data (time to extubation in ICU, LOS ICU, etc.) could have also been affected by variability in ICU provider practice. Certain providers may not have been as aggressive as other providers in extubating patients. In addition, there are other factors that affect duration of endotracheal intubation, such as failure to extubate due to opioid induced apnea. All these variables are not factored into this study.
If future prospective trials confirm the value of intraoperative MAP/mPAP ratio patterns for postoperative outcome prediction, the question will arise if MAP/mPAP ratio manipulation may be able to alter the outcome after major surgery. Increasing MAP with vasopressor/inotropic medications or lowering mPAP with pulmonary vasodilators could be beneficial.
In conclusion, the data of this retrospective study raises awareness of the mean systemic to mean pulmonary artery pressure ratio during surgery as a potential indicator for poor patient outcome following OLT. To further delineate the significance of this parameter, a multi-center, randomized, blinded prospective study with more frequent measurement points is needed for validation.
The project was presented in part at the Annual Meeting of the International Anesthesia Research Society in May, 2016.
This study provides relevant information to identify patients at risk for complications after orthotopic liver transplantation.
With the scarcity of available livers for transplantation, it is crucial that patients are properly selected and every effort is made to have the best possible outcome after the procedure.
The study is offering useful information for patient selection; the next step would be (after validation) to assess if intraoperative manipulation of this parameter would optimize patient outcomes.
This study should be of interest to any care provider involved with the care of potential liver transplant recipients.
Rebel et al described that the systemic to pulmonary artery pressure ratio can be a predictor of survival after liver transplantation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Baddour N, Hilmi I, Sugawara Y, Tanaka N S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Tuman KJ, McCarthy RJ, March RJ, Najafi H, Ivankovich AD. Morbidity and Duration of ICU stay after Cardiac Surgery. A Model for Preoperative Risk Assessment. Chest. 1992;102:36-44. [PubMed] [Cited in This Article: ] |
| 2. | Robitaille A, Denault AY, Couture P, Belisle S, Fortier A, Guertin MC, Carrier M, Martineau R. Importance of relative pulmonary hypertension in cardiac surgery: the mean systemic to pulmonary artery pressure ratio. J Cardiothor Vasc Anesth. 2006;20:331-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Kaw R, Pasupuleti V, Deshpande A, Hamieh T, Walker E, Minai OA. Pulmonary hypertension: An important predictor of outcomes in patients undergoing non-cardiac surgery. Respir Med. 2011;105:619-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Ramakrishna G, Sprung J, Barugur BS, Chandrasekaran K, McGoon MD. Impact of pulmonary hypertension on the outcomes of noncardiac surgery. J Am Coll Cardiol. 2005;45:1691-1699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Chen Y, Chan AC, Chan SC, Chok SH, Sharr W, Fung J, Liu JH, Zhen Z, Sin WC, Lo CM. A detailed evaluation of cardiac function in cirrhotic patients and its alteration with or without liver transplantation. J Cardiol. 2016;67:140-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Kia L, Shah SJ, Wang E, Sharma D, Selvaraj S, Medina C, Cahan J, Mahon H, Levitsky J. Role of pretransplant echocardiographic evaluation in predicting outcomes following liver transplantation. Am J Transplant. 2013;13:2395-2401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Fukazawa K, Yamada Y, Gologorsky E, Arheart K, Pretto EA. Hemodynamic Recovery Following Postreperfusion Syndrome in Liver Transplantation. J Cardiothor Vasc Anesth. 2014;28:994-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Ruiz-del-Arbol L, Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol. 2015;21:11502-11521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 96] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Schrier RW, Shchekochikhin D, Gines P. Renal failure in cirrhosis: prerenal azotemia, hepatorenal syndrome and acute tubular necrosis. Nephrol Dial Transplant. 2012;27:2625-2628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Van Wagner LB, Lapin B, Levitsky J, Wilkins JT, Abecassis MM, Skaro AI, Lloyd-Jones DM High early cardiovascular mortality after liver transplantation. Liver Transpl. 2014;20:1306-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Van Wagner LB, Serper M, Kang R, Levitsky J, Hohmann S, Abecassis M, Skaro A, Lloyd-Jones DM. Factors associated with major adverse cardiovascular events after liver transplantation among a national sample. Am J Transplant. 2016;16:2684-2694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Piazza NA, Singal AK. Frequency of Cardiovascular Events and Effect on Survival in Liver Transplant Recipients for Cirrhosis Due to Alcoholic or Nonalcoholic Steatohepatitis. Exp Clin Transplant. 2016;14:79-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Waxman AB, Farber HW. Using Clinical Trial End Points to Risk Stratify Patients with Pulmonary Arterial Hypertension. Circulation. 2015;132:2152-2161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Therrien J, Dore A, Gersony W, Iserin L, Liberthson R, Meijboom F, Colman JM, Oechslin E, Taylor D, Perloff J. CCA Consensus Conference 2001 update: Recommendations for the management of adults with congential heart disease. Can J Cardiol. 2001;17:940-959. [PubMed] [Cited in This Article: ] |
| 15. | Gomez CM, Palazzo MG. Pulmonary artery catheterization in anaesthesia and internsive care. Br J Anaesth. 1998;81:945-956. [PubMed] [Cited in This Article: ] |
| 16. | Bushyhead D, Kirkpatrick JN, Goldberg D. Pretransplant Echocardiographic Parameters as Markers of Posttransplant Outcomes in Liver Transplant Recipients. Liver Transpl. 2016;22:316-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Carricart M, Denault AY, Couture P, Limoges P, Babin D, Levesque S, Fortier A, Pellerin M, Tardif JC, Buithieu J. Incidence and significance of abnormal hepatic venous Doppler flow velocities before cardiac surgery. J Cardiothorac Vasc Anesth. 2005;19:751-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |