Published online Oct 8, 2016. doi: 10.4254/wjh.v8.i28.1157
Peer-review started: March 7, 2016
First decision: April 15, 2016
Revised: July 22, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: October 8, 2016
Processing time: 209 Days and 10 Hours
Primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC) and autoimmune hepatitis (AIH) constitute the classic autoimmune liver diseases (AILDs). While AIH target the hepatocytes, in PBC and PSC the targets of the autoimmune attack are the biliary epithelial cells. Persistent liver injury, associated with chronic AILD, leads to un-resolving inflammation, cell proliferation and the deposition of extracellular matrix proteins by hepatic stellate cells and portal myofibroblasts. Liver cirrhosis, and the resultant loss of normal liver function, inevitably ensues. Patients with cirrhosis have higher risks or morbidity and mortality, and that in the decompensated phase, complications of portal hypertension and/or liver dysfunction lead to rapid deterioration. Accurate diagnosis and monitoring of cirrhosis is, therefore of upmost importance. Liver biopsy is currently the gold standard technique, but highly promising non-invasive methodology is under development. Liver transplantation (LT) is an effective therapeutic option for the management of end-stage liver disease secondary to AIH, PBC and PSC. LT is indicated for AILD patients who have progressed to end-stage chronic liver disease or developed intractable symptoms or hepatic malignancy; in addition, LT may also be indicated for patients presenting with acute liver disease due to AIH who do not respond to steroids.
Core tip: In chronic liver disease, including autoimmune liver diseases, perpetual liver injury leads to persistent inflammation, cell proliferation and the deposition of extracellular matrix proteins. If left untreated, this process eventually leads to the development of liver cirrhosis, characterised by the presence of fibrosis and nodular regeneration. Liver biopsy is currently the gold standard technique, but highly promising non-invasive methodology is under development.
- Citation: Liberal R, Grant CR. Cirrhosis and autoimmune liver disease: Current understanding. World J Hepatol 2016; 8(28): 1157-1168
- URL: https://www.wjgnet.com/1948-5182/full/v8/i28/1157.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i28.1157
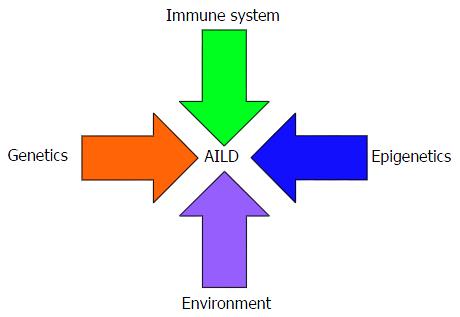
Liver disorders with probable autoimmune aetiology include autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC). Each disease complies with, to varying extents, a proposed “multiple hit hypothesis” accounting for autoimmunity development, in which interacting environmental, infectious, genetic, epigenetic and immunological factors account for the loss of tolerance to self-constituents[1]. While AIH target the hepatocytes, in PBC and PSC the targets of the autoimmune attack are the biliary epithelial cells. Each of the autoimmune liver diseases (AILDs) is associated with distinct epidemiological and clinical characteristics. However, overlap syndromes, characterised by the coexistence of features of more than one AILD, are increasingly being recognised[2].
PBC is a cholestatic autoimmune liver disease characterised by progressive destruction of the small and intermediate-sized bile ducts[3]. The histologic picture of PBC involves non-suppurative cholangitis with destruction of the biliary epithelium and portal infiltration of inflammatory cells. PBC also presents with biochemical evidence of cholestasis. PBC has pronounced female preponderance and a strong tendency to present in middle age[3]. Epidemiological characteristics of PBC are outlined in Table 1.
| PBC | PSC | AIH | |
| Female/male ratio | 10/1 | 1/2 | 4/1 |
| Average age at presentation | 50 | 41 | Childhood/adolescence and approximately 40 |
| Incidence | 0.33-5.8/100000 | 0-1.3/100000 | 0.08-3/100000 |
| Prevalence | 1.91-40.2/100000 | 0-16.2/100000 | 11.6-35.9/100000 |
| Risk within family | 1st degree relative incidence 4%-6% | Unknown | Unknown |
| Concordance in monozygotic twins | 60% | Only case reports | Only case reports |
| Note | AMA positivity | Frequent association with IBD Increased risk of hepatobiliary/colorectal malignancies | Positivity for ANA and or SMA (AIH type-1) or anti-LKM-1 (AIH type-2) |
High titre positivity for serum anti-mitochondrial autoantibodies (AMAs) is pathognomonic for PBC, being detected in up to 95% of patients[3-5]. Moreover, asymptomatic people with AMA-positivity eventually progress to disease development[6]. AMAs target lipoylated domains of the 2-oxoacid dehydrogenase complexes, with the immunodominant epitope belonging to the E2 components of the pyruvate dehydrogenase complex[3,4,7]. PBC-specific anti-nuclear autoantibodies (ANAs), with a characteristic “multiple nuclear dot” or “nuclear membrane” pattern, are found in 25%-40% of patients[8].
There is mounting evidence that the development of PBC can be accounted for by a proposed “multiple hit” hypothesis for the development of autoimmunity (Figure 1). The molecular mimicry hypothesis postulates that microorganisms with epitopes that are structurally similar to self-components trigger an immune response with interspecies promiscuity. Several potential infectious triggers have been proposed[9] including Escherichia coli[10-14] and Nosphingobium aromaticivorans[15-17].
Numerous lines of evidence demonstrate that genetic factors alter susceptibility to PBC development. Female relatives of patients are at increased risk of developing PBC, and there is a high concordance rate between monozygotic twins[18]. Strong genetic associations lying within the MHC, for example HLA-DR8 in Europe and North America, have consistently been reported[19,20]. Genome wide association studies (GWAS) have revealed non-MHC gene associations that could be related to abnormal immune activation, including IL12A, IL12RB2, STAT-4 and CTLA-4[21-23].
Ursodeoxycholic acid (UDCA) is the standard treatment for PBC, improving both biochemical and histological indicators of disease activity and elongating transplant-free survival time in a significant proportion of patients[24,25].
PSC is a chronic inflammatory disease of the biliary epithelium, characterised by progressive bile duct destruction. The small, medium and large bile ducts are affected by obliterative concentric fibrosis which leads to the development of biliary strictures[26]. In contrast to the other AILDs, PSC affects males more commonly than females[27]. The median age of onset is approximately 41 years of age[27] (Table 1).
The most common biochemical abnormality in PSC patients is elevated serum alkaline phosphatase (AP)[28]. The most reliable diagnostic tool is cholangiography, which enables visualisation of characteristic multifocal strictures within the intra- and extra-hepatic bile ducts[29]. Concomitant inflammatory bowel disease (IBD), most frequently ulcerative colitis, is found in up to 80% of patients[28,30].
As with the other AILDs, the aetiology of PSC remains unknown but it is likely to follow the proposed multiple hit hypothesis (Figure 1), resulting from interplay between numerous genetic and environmental factors. The strong link with IBD has led to the emergence of the gut/lymphocyte homing hypothesis, which postulates that memory lymphocytes primed in the gut-associated lymphoid tissue, and therefore expressing the gut-homing integin α4β7 and the chemokine receptor CCR9, migrate from the gastrointestinal tract to the liver[31,32]. Importantly, the ligand for α4β7, MAdCAM-1, and the cognate chemokine for CCR9, CCL25, both usually restricted to the gut[32,33], are aberrantly expressed in the portal vein endothelium and sinusoidal endothelium respectively in PSC patients. Moreover, approximately 20% of liver-infiltrating T cells express α4β7 and CCR9, and have an effector memory phenotype[34,35]. The “leaky gut hypothesis”, on the other hand, involves direct translocation of intestinal flora via the portal vein[28]. Although direct evidence of this phenomenon is lacking[36], future studies investigating the influence of the gut microbiota on PSC development/progression are warranted.
Similarly to the other AILDs, the strongest PSC genetic associations lie within HLA gene. In GWAS, the strongest association signals have been found near HLA-B[37-39]. There are, however, also believed to be HLA class II susceptibility genes contributing to the association signal found within in this region[38,39]. Non HLA associations identified by GWAS include BCL2LII, which encodes the pro-apoptotic protein BIM, TNFRSF14 and IL2RA[37-39].
AIH is a progressive inflammatory disease which, in contrast to the two cholestatic AILDs, targets the hepatocytes themselves. AIH has marked female predilection. AIH can present at all ages, but the two peak ages of incidence are in childhood or adolescence and at around 40 years of age[40] (Table 1). Trademark biochemical/serological characteristics of AIH are elevated aminotransferase levels, positivity for autoantibodies and increased IgG. A histological picture of interface hepatitis is typical of AIH. Autoantibody positivity is an important clinical feature of AIH, facilitating diagnosis and enabling distinction between two types of the disease. Patients seropositive for ANA and/or anti-smooth muscle autoantibodies (SMA) have AIH type-1 whereas those presenting with positivity for anti-liver kidney type-1 autoantibody (anti-LKM-1) or anti-liver cytosol type-1 (anti-LC-1) have AIH type-2[41,42].
Although AIH aetiology remains to be elucidated, available evidence is strongly suggestive of interplay between genetic and environmental factors (Figure 1). The observation that the hepatitis C virus shares high sequence homology with the auto-antigenic target of anti-LKM-1 autoantibodies, cytochrome P450-2D6, has led to the suggestion that molecular mimicry could trigger AIH development in a genetically predisposed host[43,44]. Other potential triggers for AIH include the hepatitis B virus, cytomegalovirus and the herpes simplex virus[43].
Genetic associations affecting susceptibility to disease development, response to therapy and prognosis have been reported[45]. The most significant genetic associations lie within the MHC, at the HLA-DRB1 locus. Susceptibility to AIH type-1 is linked to alleles encoding the HLA-DR3 and DR4 molecules[46], while AIH-2 susceptibility and severity have been linked to alleles encoding the HLA-DR3 and DR7 molecules[47]. Susceptibility to AIH has also been linked to polymorphisms in genes located outside the MHC, including CTLA-4[48], TNF-α[49] and Fas[50].
With the standard treatment regimen for AIH - prednisolone, with or without the addition of azathioprine - up to 80% of AIH patients are able to reach remission[51].
It is not uncommon for patients to present with features characteristic of AIH and either PSC or PBC. Because standardised and validated diagnostic criteria are lacking, these “overlap syndromes” remain ill defined. PBC/AIH overlap is present in some 10% of AIH or PBC patients[52,53], and the most commonly used method for diagnosis is the presence of two of the following features of AIH in conjunction with two of the following features of PBC. The AIH features are: (1) ALT at least 5 times the upper limit of normal (ULN); (2) SMA positivity or IgG level of at least 2 times ULN; and (3) liver histology showing moderate or severe periportal or periseptal inflammation. The PBC criteria are: (1) AP at least twice ULN or gamma glutamyl transferase above 5 times ULN; (2) AMA positivity; and (3) bile duct lesions on liver biopsy[52,54,55]. AIH/PSC overlap is now believed to represent a significant proportion of patients with AILD[56,57]. The characteristics of AIH/PSC overlap are the classical features of AIH-1 - positivity for ANA and/or SMA, high IgG levels and interface hepatitis on biopsy - in addition to biochemical evidence of cholestasis, frequent occurrence of IBD, histological features consistent with PSC[58]. Cholangiographic evidence of intrahepatic or extrahepatic PSC also supports this diagnosis[59].
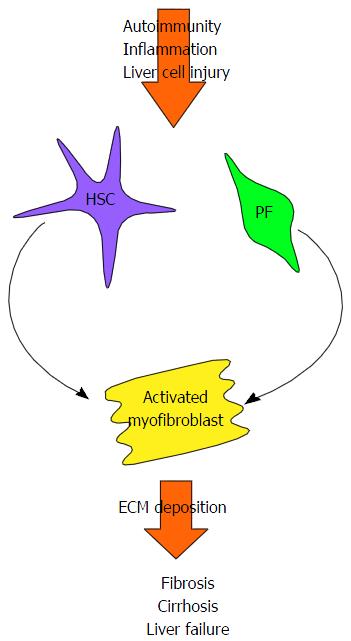
In chronic liver disease, including AILD, perpetual liver injury leads to persistent inflammation, cell proliferation and the deposition of extracellular matrix proteins. If left untreated, this process eventually leads to the development of liver cirrhosis, characterised by nodular regeneration diffuse nodular regeneration surrounded by fibrotic septa with consequent extinction of the parenchyma, together leading to distortion of hepatic vascular architecture[60]. Loss of normal liver function inevitably ensues (Figure 2)[61].
Hepatic stellate cells, found in the space of Dissé, have long been believed to be the main contributors to liver fibrosis. Liver damage induces hepatic stellate cells to differentiate into proliferative and contractile myofibroblasts, with a pro-inflammatory and fibrogenic phenotype[61-63]. Portal fibroblasts, located in the connective tissue of the portal triad are another source of myofibroblasts[64]. These are of particular importance in the context of the cholestatic AILDs. Liver damage leads to the myofibroblastic differentiation of quiescent portal fibroblasts[64], a process which can be enhanced in these conditions by the cholangiocytes themselves. When cholangiocytes become “reactive”, they proliferate and express co-stimulatory molecules, chemokines and pro-fibrogenic molecules, therefore further promoting fibrogenesis[65-70]. Bile acids, elevated as a consequence of cholestasis, could also perpetuate fibrogenesis indirectly by damaging hepatocytes[71], or by directly targeting myofibroblasts[72].
It has also been suggested that hepatic myofibroblasts could arise from hepatocytes or cholangiocytes via epithelial-mesenchymal transition, whereby polarised epithelial cells undergo phenotypic transformation in response to microenvironmental cues[73]. There are reports that hepatic epithelial cells can acquire some of the phenotypic characteristics of myofibroblastic cells in vitro. Co-expression of epithelial and fibroblastic cell markers has also been described in human tissue sections[74,75]. However, partly because these cell “markers” inadequately define both the epithelial and fibroblastic populations, conclusive evidence of epithelial-mesenchymal transition has been hard to come by. Furthermore, lineage tracing studies, using Cre/lox recombination, have failed to find evidence of liver epithelial cell-mesencymal transition in murine models of bile-duct ligation or hepatitis induced by carbon tetrachloride (CCL4) or 3,5-diethoxycarbonyl-1,4-dihydrocollidine[76,77].
It is well known that, compared with pre-cirrhotic patients, patients with cirrhosis have higher risks or morbidity and mortality[78]. Cirrhosis can be divided into a compensated phase, free of symptoms, and a decompensated phase, in which complications of portal hypertension and/or liver dysfunction lead to rapid deterioration. The two stages can be considered separate clinical entities according to the AASLD and EASL guidelines[79]. Median survival time in the compensated phase is over 12 years, whereas survival in the decompensated phase drops to approximately 2 years. The decompensated phase is defined by the development of jaundice, ascites, variceal haemorrhage or encephalopathy[80,81]. The compensated stage has been further divided into stage 1, consisting of patients lacking varices, and stage 2, characterised by the presence of varices in the absence or variceal bleeding. The decompensated stage has been split into stage 3, associated with ascites and a lack of variceal haemorrhage, and stage 4, comprising patients with variceal haemorrhage (with or without ascites). One year mortality rates of 1%, 3%, 20% and 57% respectively have been reported[82,83]. In a recent study, however, Zipprich et al[84] (2012) failed to replicate entirely these reported values, finding that stage 3 and stage 4 patients had one year survival rates of approximately 20% and 18% respectively. The authors of this study cite recent advances in variceal haemorrhage therapy[85] as a potential reason for this discrepancy and proposed modifications to the system of stratification. The newly defined stage 3 consists of patients with variceal haemorrhage but without ascites, while stage 4 is characterised by the presence of ascites (with or without variceal bleeding)[84].
The risk of progression from compensated to decompensated cirrhosis is approximately 31% in the first year of diagnosis and 5%-7% thereafter[86]. Because of the striking reduction in survival time in the decompensated state, it is important to identify patients at greatest risk of cirrhosis progression. Newly developed non-invasive techniques for fibrosis/cirrhosis assessment are currently being tested.
The Child-Pugh, and more recently developed Model of End-Stage Liver Disease (MELD) Scores are the most widely used methods by which prognosis is assessed in the context of end-stage liver disease. The Child-Pugh Score incorporates values between 1 and 3 for each of the following criteria: Degree of encephalopathy, presence of ascites, serum bilirubin and albubin levels and international normalised ratio (INR). The MELD score encompasses bilirubin, INR and creatinine levels[87]. MELD was initially developed for predicting survival following transhepatic portosystemic shunt, but is now used to accurately predict survival in the context of cirrhosis[88], list patients for transplant and allocate organs.
Liver biopsy is still the most accurate and widely used method by which cirrhosis can be diagnosed and staged. There are, however, notable disadvantages to this method of examination, including cost, risk of bleeding, and sampling error[89]. Non-invasive tests for both diagnosis and assessment of fibrosis/cirrhosis progression are becoming increasingly sought. Proposed tests include those using the results of routine liver-function examinations, such as the AST-to-platelet ratio index, as well as examinations to measure liver stiffness; FibroTest and transient elastography (TE; FibroScan)[90,91]. There are promising indications that non-invasive methods could be used in the context of AILD. In PBC, liver stiffness tests show high performance in diagnosing significant fibrosis, severe fibrosis and cirrhosis. Progression of liver stiffness has also been used as an accurate measure of overall prognosis in PBC[92-94]. The addition of serological markers to the liver stiffness score does not appear to improve test outcome[93]. In a study also involving both PBC and PSC patients, liver stiffness was also shown to correlate with progression of fibrosis and histological scores[95]. Using a cohort of 404 patients with varied liver diseases, including PBC, PSC and AIH, Malik et al[96] (2010) found that liver stiffness scores accurately identified patients with compensated cirrhosis. Although highly promising, these results, particularly in the context of AIH, need to be confirmed in larger cohorts of patients.
PBC progresses through a number of stages: Preclinical, asymptomatic, symptomatic, and liver failure. The preclinical phase is symptom-free and is associated with AMA positivity in the absence of biochemical indications of liver disease[6,97]. Biochemical abnormalities eventually appear after a median time of 5.6 years (range, 1-20 years)[6], but this phase is not yet associated with the presence of symptoms. When symptoms eventually develop, they are most commonly fatigue and pruritus, and later varices, oedema or ascites.
Liver failure is characterised by the accelerated development of jaundice, and is associated with poor prognosis[98]. Mean survival for patients with a bilirubin of 2.0 mg/dL is 4 years, while for those with bilirubin of 6.0 mg/dL is only 2 years[98]. PBC prognosis has dramatically improved in the last 20 years thanks to earlier diagnosis and the introduction of UDCA as the mainstay of treatment[99,100].
UDCA slows fibrosis progression and delays cirrhosis development[101]. In clinical trials, UDCA treatment of PBC patients decreased the development of oesophageal varices and prolonged survival[102-106]. Cirrhosis does, however, still develop in UDCA-treated PBC patients[107]. Indeed, the development of cirrhosis under UDCA treatment is an independent predictor of negative outcome[101,107].
Histologically, PBC can be divided according to the presence of fibrosis/cirrhosis into four stages[108,109]. Stage one is characterised by portal inflammatory cell infiltrate, which, in stage two, invades the liver parenchyma. In stage three, bridging fibrosis, in which fibrotic septa extend from and link the portal tracts, can be seen. Stage four is characterised by progression to cirrhosis[109]. The development of cirrhosis does not occur uniformly throughout the liver, thus features of all four stages can ocur simultaneously in a single biopsy specimen. Histological staging should depend upon the most advanced histological features[25].
Histological stages can predict survival of PBC patients[110]. In untreated PBC patients, the median time to the development of extensive fibrosis is 2 years. The probability of remaining in early stages after 4 years is 29%, whereas development of cirrhosis occurs in 50% of patients originally demonstrating histological evidence of interface hepatitis without fibrosis[111]. In two studies the proportion of patients developing liver failure during a follow-up time of 5 years was found to be 15%[112] and 25%[113]. The development of oesophageal varices, and the associated impact on survival, has been examined in a prospective study over the course of 5.6 years, which included 256 patients[114]. Twenty-eight percent of patients were cirrhotic. Nearly one-third of patients developed oesophageal varices, after which the 3-year survival was 59%. Survival after the first bleeding episode was 46%[114].
The introduction of UDCA as first line treatment for PBC patients has changed the natural history of the disease[25,100,115,116]. Indeed, the number of PBC patients requiring liver transplantation (LT) decreased by 20% in between 1996 and 2006[115]. Additionally, PBC has fallen in the ranking of the most common indications for LT from the first to the sixth place LT over a period of 20 years[25].
Several papers have also assessed the impact of UDCA therapy on the progression rate of cirrhosis in PBC patients. Corpechot et al[107] examined progression to cirrhosis in 183 UDCA-treated PBC patients. In this study, 21% of patients developed cirrhosis during follow-up. The incidence of cirrhosis in patients followed up from stages 1, 2 and 3 was 4%, 12% and 59% respectively and the median length of times to cirrhosis development was 25, 20 and 4 years respectively. Albumin and bilirubin levels, and the histological severity of interface hepatitis were independently associated with progression to cirrhosis; cirrhosis was most likely to develop in patients with serum bilirubin over 17 μmol/L, serum albumin below 38 g/L and in patients with moderate to severe interface hepatitis[107]. The impact of UDCA treatment oesophageal varices development has been examined in a 4-year prospective study including patients who received UDCA vs patients who received placebo. In the UDCA arm, the risk of varices development was 16%, while for those in the placebo group was 58%[103].
Typical symptoms of PSC, occurring in a variable number of patients include pruritus, abdominal pain, malaise, weight loss, and episodes of fever and chills[117]. About 50% of PSC patients will present symptomatically[118,119]. Similarly to PBC, PSC progresses through four histological stages[120]. In stage 1, which is known as the portal stage, changes are restricted to the portal tracts with features of mild hepatitis and cholangitis. Stage 2, known as the periportal stage, is characterised by extension of the lesion to include periportal fibrosis and occasionally interphase hepatitis. In this phase, the portal tracts are often notably enlarged. By stage 3, the septal stage, bridging fibrous septa have developed and the bile ducts have begun to degenerate and disappear. Stage 4 is characterised by cirrhosis[120]. The rate of progression through these stages has been investigated. Of PSC patients in the periportal stage, 42%, 66% and 93% progressed over 1, 2 and 5 years respectively. Of patients in the septal stage, 14%, 25% and 52% progressed over 1, 2 and 5 years respectively. In 15% of total observations, regression of histologic stage could be observed, highlighting the problem of sample variability when serial liver biopsies are used during the period of follow-up[121].
PSC can present at later stages of disease development, with complications of cirrhosis and portal hypertension[122]. Similarly to other causes of cirrhosis, portal hypertension gradually develops in cirrhotic PSC patients[119]. In one study, 36% of 283 newly diagnosed PSC patients had varices[123].
In a cohort of over 450 AIH patients, 30% had evidence of cirrhosis at diagnosis, with a further 10% developing cirrhosis during a median follow-up time of 7.2 years. The presence of cirrhosis at diagnosis correlated with negative outcome (LT or death)[124]. In another study, including 126 AIH patients, Feld et al[125] (2005) reported that 33% of patients had histological evidence of cirrhosis at diagnosis. With the exception of platelet count, which was lower in patients with cirrhosis, laboratory parameters, patient demographics and AIH scores did not differ between cirrhotic and non-cirrhotic patients. A similar frequency of patients from each group were symptomatic at diagnosis and an equivalent proportion had good response to treatment[125]. Importantly, similar response to treatment has also been reported elsewhere[126]. Feld et al[125] (2005) also found, however, that the presence of cirrhosis significantly increased risk of progression to LT or death. Consistent with the above studies, Verma et al[127] (2004) reported that 28% of AIH patients were cirrhotic at diagnosis. In this study, a further 20% of patients developed cirrhosis during 52 mo of follow-up. Again, cirrhosis was an independent predictor of poor outcome in this cohort[127]. On the other hand, studies in the adult[126,128] and paediatric[129] settings, of comparable size and methodology to those described above, have not found associations between the presence of cirrhosis at diagnosis and the likelihood of poor outcome.
In one study, patients diagnosed between the ages of 21 and 60 years of age were more likely to present with cirrhosis than those outside of this range. Male patients were also more likely to have cirrhosis compared to their female counterparts. Low serum albumin concentrations, prolonged INR and low platelet count were all more frequently associated with the cirrhotic group of AIH patients[130].
There are indications that cirrhosis is more common among AIH type-1 patients compared to patients with type-2 AIH. In a paediatric study, 69% of ANA/SMA positive patients had evidence of “definite cirrhosis” on initial biopsy, whereas only 38% of patients positive for anti-LKM-1 were cirrhotic. On follow-up these values increased to 74% and 44% respectively[131].
LT is indicated for AILD patients who have progressed to end-stage chronic liver disease or developed intractable symptoms or hepatocellular carcinoma (HCC)[132,133]; in addition, LT may also be indicated for patients presenting with acute liver disease due to AIH who do not respond to steroids[134]. In total, AILDs accounts for almost one fourth of LTs performed in the United States and in Europe[135].
The indications for LT in PBC are, for the most part, identical to those in patients with end-stage chronic liver disease of other aetiology[100,132]. The majority of transplants occur due to end-stage chronic liver disease when the MELD score is higher than 16[136]. Other indications for LT include HCC, portopulmonary hypertension or hepato-pulmonary syndrome[137]. Other than this, few PBC patients with non-cirrhotic portal hypertension associated with obliterative portal venopathy or nodular regenerative hyperplasia will benefit from transplant[138]. Finally, even when liver function is sufficient[139], LT may be indicated if intractable symptoms, most notable refractory pruritus, are present[137,140].
The immunosuppressive regimen most commonly used following LT is a combination of corticosteroids, which are withdrawn over a period of three months, a calcineurin inhibitor (CNI) and mycophenolate mofetil or azathioprine. This regimen has a very successful outcome[136]; with 1, 3 and 5 year patient survivals of 94%, 91% and 82% respectively, and graft survivals of 85%, 83% and 75% respectively[141]. Analysis of the UNOS database showed that PBC living donor transplant recipients had estimated 1, 3 and 5 year patient survivals of 93%, 90% and 86% and deceased donor transplant recipients had estimated survivals of 90%, 87% and 85% respectively. Estimated graft survivals at 1, 3 and 5 years for living donor LT was 86%, 81% and 77% respectively, and for deceased donor LT was 85%, 83% and 81% respectively[142].
Similarly to PBC, and other liver diseases associated with cirrhosis, LT is indicated in PSC patients with end-stage liver disease (i.e., with a MELD score above 16)[143,144]. HCC can occur in PSC patients with cirrhosis, and in this context, LT prioritisation follows the same rule as that for other cirrhotic patients with HCC[137,145]. In PSC, LT may also be indicated in patients with intractable pruritus or those with recurrent bacterial cholangitis, and limited stage cholangiocarcinoma[118,122,143].
LT for PSC usually has good outcome[139]. In one report, the 1, 2 and 5 year actuarial patient survivals for LT for PSC were 90%, 86% and 85%, and graft survivals were 82%, 77% and 72% respectively[146]. In a study from the Mayo Clinic comprising 150 transplanted PSC cases, similar patient survival at 1, 2, 5 and 10 years of 94%, 92%, 86% and 70%, and graft survival of 83%, 83%, 79% and 61% was reported[147].
Overall, AIH accounts for some 3% of paediatric and up to 6% of adult LTs[40]. The natural course of AIH is understood mostly thanks to the last placebo-controlled trials published 4 decades ago[148-150]. These reports demonstrated that, without treatment, AIH patients have poor survival with 40% of deaths within 6 mo from diagnosis. With treatment, 10-year survival rate of AIH patients is over 80%[125,151].
LT is indicated for AIH patients presenting with acute liver failure who do not respond to steroids, for those patients with advanced cirrhosis and for those with HCC[136,152].
The immunosuppressive strategy most commonly adopted consists in the combination of prednisolone and a CNI[153], leading to excellent outcome with 5 and 10 year patient survivals of 90% and 75%[51], and 1 and 5 year graft survivals of 84% and 75%[51,154].
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gonzalez-Reimers E, Vij M, Weng HL S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Bogdanos DP, Gershwin ME. What is new in primary biliary cirrhosis? Dig Dis. 2012;30 Suppl 1:20-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 324] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 3. | Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 965] [Cited by in F6Publishing: 893] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 4. | Bogdanos DP, Baum H, Vergani D. Antimitochondrial and other autoantibodies. Clin Liver Dis. 2003;7:759-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Bogdanos DP, Komorowski L. Disease-specific autoantibodies in primary biliary cirrhosis. Clin Chim Acta. 2011;412:502-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, James OF. Natural history of early primary biliary cirrhosis. Lancet. 1996;348:1399-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 266] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 202] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol. 2013;8:303-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Smyk DS, Rigopoulou EI, Bogdanos DP. Potential Roles for Infectious Agents in the Pathophysiology of Primary Biliary Cirrhosis: What’s New? Curr Infect Dis Rep. 2013;15:14-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Bogdanos DP, Baum H, Grasso A, Okamoto M, Butler P, Ma Y, Rigopoulou E, Montalto P, Davies ET, Burroughs AK. Microbial mimics are major targets of crossreactivity with human pyruvate dehydrogenase in primary biliary cirrhosis. J Hepatol. 2004;40:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Burroughs AK, Rosenstein IJ, Epstein O, Hamilton-Miller JM, Brumfitt W, Sherlock S. Bacteriuria and primary biliary cirrhosis. Gut. 1984;25:133-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 156] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Shigematsu H, Shimoda S, Nakamura M, Matsushita S, Nishimura Y, Sakamoto N, Ichiki Y, Niho Y, Gershwin ME, Ishibashi H. Fine specificity of T cells reactive to human PDC-E2 163-176 peptide, the immunodominant autoantigen in primary biliary cirrhosis: implications for molecular mimicry and cross-recognition among mitochondrial autoantigens. Hepatology. 2000;32:901-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe EB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831-1840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Shimoda S, Nakamura M, Shigematsu H, Tanimoto H, Gushima T, Gershwin ME, Ishibashi H. Mimicry peptides of human PDC-E2 163-176 peptide, the immunodominant T-cell epitope of primary biliary cirrhosis. Hepatology. 2000;31:1212-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Mohammed JP, Fusakio ME, Rainbow DB, Moule C, Fraser HI, Clark J, Todd JA, Peterson LB, Savage PB, Wills-Karp M. Identification of Cd101 as a susceptibility gene for Novosphingobium aromaticivorans-induced liver autoimmunity. J Immunol. 2011;187:337-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Olafsson S, Gudjonsson H, Selmi C, Amano K, Invernizzi P, Podda M, Gershwin ME. Antimitochondrial antibodies and reactivity to N. aromaticivorans proteins in Icelandic patients with primary biliary cirrhosis and their relatives. Am J Gastroenterol. 2004;99:2143-2146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250-1257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, Gordon SC, Wright HI, Zweiban B, Podda M. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 378] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 19. | Donaldson PT, Baragiotta A, Heneghan MA, Floreani A, Venturi C, Underhill JA, Jones DE, James OF, Bassendine MF. HLA class II alleles, genotypes, haplotypes, and amino acids in primary biliary cirrhosis: a large-scale study. Hepatology. 2006;44:667-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Invernizzi P, Selmi C, Poli F, Frison S, Floreani A, Alvaro D, Almasio P, Rosina F, Marzioni M, Fabris L. Human leukocyte antigen polymorphisms in Italian primary biliary cirrhosis: a multicenter study of 664 patients and 1992 healthy controls. Hepatology. 2008;48:1906-1912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, Gu X, Walker EJ, Jing K, Juran BD. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544-2555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 456] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 22. | Liu X, Invernizzi P, Lu Y, Kosoy R, Lu Y, Bianchi I, Podda M, Xu C, Xie G, Macciardi F. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42:658-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 307] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 23. | Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, Heneghan MA, Neuberger JM, Donaldson PT, Day DB. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 371] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 24. | Lindor K. Ursodeoxycholic acid for the treatment of primary biliary cirrhosis. N Engl J Med. 2007;357:1524-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 872] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 26. | Portmann B, Zen Y. Inflammatory disease of the bile ducts-cholangiopathies: liver biopsy challenge and clinicopathological correlation. Histopathology. 2012;60:236-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Molodecky NA, Kareemi H, Parab R, Barkema HW, Quan H, Myers RP, Kaplan GG. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53:1590-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 28. | Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382:1587-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 29. | MacCarty RL, LaRusso NF, Wiesner RH, Ludwig J. Primary sclerosing cholangitis: findings on cholangiography and pancreatography. Radiology. 1983;149:39-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 201] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Boonstra K, van Erpecum KJ, van Nieuwkerk KM, Drenth JP, Poen AC, Witteman BJ, Tuynman HA, Beuers U, Ponsioen CY. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2270-2276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Förster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 536] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 32. | Trivedi PJ, Adams DH. Mucosal immunity in liver autoimmunity: a comprehensive review. J Autoimmun. 2013;46:97-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97-110. [PubMed] [Cited in This Article: ] |
| 34. | Grant AJ, Lalor PF, Hübscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology. 2001;33:1065-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hübscher SG, Briskin M, Salmon M, Adams DH. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 230] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Pollheimer MJ, Halilbasic E, Fickert P, Trauner M. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2011;25:727-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Karlsen TH, Franke A, Melum E, Kaser A, Hov JR, Balschun T, Lie BA, Bergquist A, Schramm C, Weismüller TJ. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138:1102-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 38. | Melum E, Franke A, Schramm C, Weismüller TJ, Gotthardt DN, Offner FA, Juran BD, Laerdahl JK, Labi V, Björnsson E. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43:17-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 39. | Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, Andreassen OA, Weersma RK, Weismüller TJ, Eksteen B. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 289] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 40. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1039] [Cited by in F6Publishing: 969] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 41. | Vergani D, Alvarez F, Bianchi FB, Cançado EL, Mackay IR, Manns MP, Nishioka M, Penner E. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41:677-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | Mieli-Vergani G, Vergani D. Paediatric autoimmune liver disease. Arch Dis Child. 2013;98:1012-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Bogdanos DP, Choudhuri K, Vergani D. Molecular mimicry and autoimmune liver disease: virtuous intentions, malign consequences. Liver. 2001;21:225-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Kerkar N, Choudhuri K, Ma Y, Mahmoud A, Bogdanos DP, Muratori L, Bianchi F, Williams R, Mieli-Vergani G, Vergani D. Cytochrome P4502D6(193-212): a new immunodominant epitope and target of virus/self cross-reactivity in liver kidney microsomal autoantibody type 1-positive liver disease. J Immunol. 2003;170:1481-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Donaldson PT. Genetics in autoimmune hepatitis. Semin Liver Dis. 2002;22:353-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Donaldson PT, Doherty DG, Hayllar KM, McFarlane IG, Johnson PJ, Williams R. Susceptibility to autoimmune chronic active hepatitis: human leukocyte antigens DR4 and A1-B8-DR3 are independent risk factors. Hepatology. 1991;13:701-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 237] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Djilali-Saiah I, Fakhfakh A, Louafi H, Caillat-Zucman S, Debray D, Alvarez F. HLA class II influences humoral autoimmunity in patients with type 2 autoimmune hepatitis. J Hepatol. 2006;45:844-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Agarwal K, Czaja AJ, Jones DE, Donaldson PT. Cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphisms and susceptibility to type 1 autoimmune hepatitis. Hepatology. 2000;31:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 49. | Cookson S, Constantini PK, Clare M, Underhill JA, Bernal W, Czaja AJ, Donaldson PT. Frequency and nature of cytokine gene polymorphisms in type 1 autoimmune hepatitis. Hepatology. 1999;30:851-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Agarwal K, Czaja AJ, Donaldson PT. A functional Fas promoter polymorphism is associated with a severe phenotype in type 1 autoimmune hepatitis characterized by early development of cirrhosis. Tissue Antigens. 2007;69:227-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 585] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 52. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 454] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 53. | Klöppel G, Seifert G, Lindner H, Dammermann R, Sack HJ, Berg PA. Histopathological features in mixed types of chronic aggressive hepatitis and primary biliary cirrhosis. Correlations of liver histology with mitochondrial antibodies of different specificity. Virchows Arch A Pathol Anat Histol. 1977;373:143-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 73] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | European Association for the Study of the L. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1227] [Cited by in F6Publishing: 1163] [Article Influence: 77.5] [Reference Citation Analysis (1)] |
| 55. | Liberal R, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 56. | Gohlke F, Lohse AW, Dienes HP, Löhr H, Märker-Hermann E, Gerken G, Meyer zum Büschenfelde KH. Evidence for an overlap syndrome of autoimmune hepatitis and primary sclerosing cholangitis. J Hepatol. 1996;24:699-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 126] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | el-Shabrawi M, Wilkinson ML, Portmann B, Mieli-Vergani G, Chong SK, Williams R, Mowat AP. Primary sclerosing cholangitis in childhood. Gastroenterology. 1987;92:1226-1235. [PubMed] [Cited in This Article: ] |
| 58. | Floreani A, Rizzotto ER, Ferrara F, Carderi I, Caroli D, Blasone L, Baldo V. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100:1516-1522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Kaya M, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary sclerosing cholangitis: an evaluation of a modified scoring system. J Hepatol. 2000;33:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1139] [Cited by in F6Publishing: 1243] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 61. | Penz-Österreicher M, Österreicher CH, Trauner M. Fibrosis in autoimmune and cholestatic liver disease. Best Pract Res Clin Gastroenterol. 2011;25:245-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3381] [Cited by in F6Publishing: 3947] [Article Influence: 207.7] [Reference Citation Analysis (3)] |
| 63. | Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438-1444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 65. | Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, Glaser SS, Francis H, Cantafora A, Blotta I. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 2005;43:875-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Cramer T, Schuppan D, Bauer M, Pfander D, Neuhaus P, Herbst H. Hepatocyte growth factor and c-Met expression in rat and human liver fibrosis. Liver Int. 2004;24:335-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Fabris L, Strazzabosco M, Crosby HA, Ballardini G, Hubscher SG, Kelly DA, Neuberger JM, Strain AJ, Joplin R. Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and Bcl-2 from primary cholangiopathies and ductal plate malformations. Am J Pathol. 2000;156:1599-1612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, Marra F, Herbst H, Alpini G, Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31:100-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Luo B, Tang L, Wang Z, Zhang J, Ling Y, Feng W, Sun JZ, Stockard CR, Frost AR, Chen YF. Cholangiocyte endothelin 1 and transforming growth factor beta1 production in rat experimental hepatopulmonary syndrome. Gastroenterology. 2005;129:682-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Rockey DC, Fouassier L, Chung JJ, Carayon A, Vallee P, Rey C, Housset C. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 71. | Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461-2483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 569] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 72. | Svegliati-Baroni G, Ridolfi F, Hannivoort R, Saccomanno S, Homan M, De Minicis S, Jansen PL, Candelaresi C, Benedetti A, Moshage H. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology. 2005;128:1042-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 73. | Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;50:2007-2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 74. | Díaz R, Kim JW, Hui JJ, Li Z, Swain GP, Fong KS, Csiszar K, Russo PA, Rand EB, Furth EE. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Hum Pathol. 2008;39:102-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 75. | Robertson H, Kirby JA, Yip WW, Jones DE, Burt AD. Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology. 2007;45:977-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 76. | Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 77. | Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, Brenner DA. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 78. | Udell JA, Wang CS, Tinmouth J, FitzGerald JM, Ayas NT, Simel DL, Schulzer M, Mak E, Yoshida EM. Does this patient with liver disease have cirrhosis? JAMA. 2012;307:832-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 79. | Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding--unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 80. | Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 682] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 81. | Saunders JB, Walters JR, Davies AP, Paton A. A 20-year prospective study of cirrhosis. Br Med J (Clin Res Ed). 1981;282:263-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 273] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1892] [Cited by in F6Publishing: 1991] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 83. | de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 794] [Cited by in F6Publishing: 715] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 84. | Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 85. | Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 86. | Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32:1343-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 87. | Wendon J, Bernal W, Willars C, Auzinger G. Critical care and cirrhosis: outcome and benefit. Curr Opin Crit Care. 2011;17:533-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3535] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 89. | Bianchi L. Liver biopsy in elevated liver functions tests? An old question revisited. J Hepatol. 2001;35:290-294. [PubMed] [Cited in This Article: ] |
| 90. | Castera L, Pinzani M. Non-invasive assessment of liver fibrosis: are we ready? Lancet. 2010;375:1419-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 91. | Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 92. | Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, Chazouillères O, Poupon R. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 93. | Floreani A, Cazzagon N, Martines D, Cavalletto L, Baldo V, Chemello L. Performance and utility of transient elastography and noninvasive markers of liver fibrosis in primary biliary cirrhosis. Dig Liver Dis. 2011;43:887-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Friedrich-Rust M, Müller C, Winckler A, Kriener S, Herrmann E, Holtmeier J, Poynard T, Vogl TJ, Zeuzem S, Hammerstingl R. Assessment of liver fibrosis and steatosis in PBC with FibroScan, MRI, MR-spectroscopy, and serum markers. J Clin Gastroenterol. 2010;44:58-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 316] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 96. | Malik R, Lai M, Sadiq A, Farnan R, Mehta S, Nasser I, Challies T, Schuppan D, Afdhal N. Comparison of transient elastography, serum markers and clinical signs for the diagnosis of compensated cirrhosis. J Gastroenterol Hepatol. 2010;25:1562-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Mayo MJ. Natural history of primary biliary cirrhosis. Clin Liver Dis. 2008;12:277-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut. 1979;20:137-140. [PubMed] [Cited in This Article: ] |
| 99. | Prince MI, James OF. The epidemiology of primary biliary cirrhosis. Clin Liver Dis. 2003;7:795-819. [PubMed] [Cited in This Article: ] |
| 100. | Selmi C, Bowlus CL, Gershwin ME, Coppel RL. Primary biliary cirrhosis. Lancet. 2011;377:1600-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 101. | Corpechot C, Carrat F, Bahr A, Chrétien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297-303. [PubMed] [Cited in This Article: ] |
| 102. | Poupon RE, Lindor KD, Parés A, Chazouillères O, Poupon R, Heathcote EJ. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39:12-16. [PubMed] [Cited in This Article: ] |
| 103. | Lindor KD, Jorgensen RA, Therneau TM, Malinchoc M, Dickson ER. Ursodeoxycholic acid delays the onset of esophageal varices in primary biliary cirrhosis. Mayo Clin Proc. 1997;72:1137-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 104. | Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology. 1996;110:1515-1518. [PubMed] [Cited in This Article: ] |
| 105. | Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 531] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 106. | Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N Engl J Med. 1994;330:1342-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 325] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 107. | Corpechot C, Carrat F, Poupon R, Poupon RE. Primary biliary cirrhosis: incidence and predictive factors of cirrhosis development in ursodiol-treated patients. Gastroenterology. 2002;122:652-658. [PubMed] [Cited in This Article: ] |
| 108. | Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 519] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 109. | Scheuer PJ. Primary biliary cirrhosis: diagnosis, pathology and pathogenesis. Postgrad Med J. 1983;59 Suppl 4:106-115. [PubMed] [Cited in This Article: ] |
| 110. | Roll J, Boyer JL, Barry D, Klatskin G. The prognostic importance of clinical and histologic features in asymptomatic and symptomatic primary biliary cirrhosis. N Engl J Med. 1983;308:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 286] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 111. | Locke GR, Therneau TM, Ludwig J, Dickson ER, Lindor KD. Time course of histological progression in primary biliary cirrhosis. Hepatology. 1996;23:52-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Prince M, Chetwynd A, Newman W, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology. 2002;123:1044-1051. [PubMed] [Cited in This Article: ] |
| 113. | Christensen E, Neuberger J, Crowe J, Portmann B, Williams R, Altman DG, Popper H, Doniach D, Ranek L, Tygstrup N. Azathioprine and prognosis in primary biliary cirrhosis. Gastroenterology. 1986;90:508-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 114. | Gores GJ, Wiesner RH, Dickson ER, Zinsmeister AR, Jorgensen RA, Langworthy A. Prospective evaluation of esophageal varices in primary biliary cirrhosis: development, natural history, and influence on survival. Gastroenterology. 1989;96:1552-1559. [PubMed] [Cited in This Article: ] |
| 115. | Lee J, Belanger A, Doucette JT, Stanca C, Friedman S, Bach N. Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:1313-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 116. | Gong Y, Huang Z, Christensen E, Gluud C. Ursodeoxycholic acid for patients with primary biliary cirrhosis: an updated systematic review and meta-analysis of randomized clinical trials using Bayesian approach as sensitivity analyses. Am J Gastroenterol. 2007;102:1799-1807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 633] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 118. | Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, Glimcher LH, Lord GM. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci USA. 2009;106:17876-17881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 119. | Mendes FD, Lindor KD. Primary sclerosing cholangitis. Clin Liver Dis. 2004;8:195-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 120. | Ludwig J. Surgical pathology of the syndrome of primary sclerosing cholangitis. Am J Surg Pathol. 1989;13 Suppl 1:43-49. [PubMed] [Cited in This Article: ] |
| 121. | Angulo P, Larson DR, Therneau TM, LaRusso NF, Batts KP, Lindor KD. Time course of histological progression in primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:3310-3313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 809] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 123. | Zein CO, Lindor KD, Angulo P. Prevalence and predictors of esophageal varices in patients with primary sclerosing cholangitis. Hepatology. 2004;39:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 124. | Werner M, Prytz H, Ohlsson B, Almer S, Björnsson E, Bergquist A, Wallerstedt S, Sandberg-Gertzén H, Hultcrantz R, Sangfelt P. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol. 2008;43:1232-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 125. | Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology. 2005;42:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 126. | Ngu JH, Bechly K, Chapman BA, Burt MJ, Barclay ML, Gearry RB, Stedman CA. Population-based epidemiology study of autoimmune hepatitis: a disease of older women? J Gastroenterol Hepatol. 2010;25:1681-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 129] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 127. | Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004;99:1510-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 128. | Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110:848-857. [PubMed] [Cited in This Article: ] |
| 129. | Radhakrishnan KR, Alkhouri N, Worley S, Arrigain S, Hupertz V, Kay M, Yerian L, Wyllie R, Feldstein AE. Autoimmune hepatitis in children--impact of cirrhosis at presentation on natural history and long-term outcome. Dig Liver Dis. 2010;42:724-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 130. | Ngu JH, Gearry RB, Frampton CM, Stedman CA. Predictors of poor outcome in patients w ith autoimmune hepatitis: a population-based study. Hepatology. 2013;57:2399-2406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 131. | Gregorio GV, Portmann B, Reid F, Donaldson PT, Doherty DG, McCartney M, Mowat AP, Vergani D, Mieli-Vergani G. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997;25:541-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 390] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 132. | Ahmed A, Keeffe EB. Current indications and contraindications for liver transplantation. Clin Liver Dis. 2007;11:227-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 133. | Liberal R, Zen Y, Mieli-Vergani G, Vergani D. Liver transplantation and autoimmune liver diseases. Liver Transpl. 2013;19:1065-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 134. | Kessler WR, Cummings OW, Eckert G, Chalasani N, Lumeng L, Kwo PY. Fulminant hepatic failure as the initial presentation of acute autoimmune hepatitis. Clin Gastroenterol Hepatol. 2004;2:625-631. [PubMed] [Cited in This Article: ] |
| 135. | Ilyas JA, O’Mahony CA, Vierling JM. Liver transplantation in autoimmune liver diseases. Best Pract Res Clin Gastroenterol. 2011;25:765-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 136. | Mottershead M, Neuberger J. Transplantation in autoimmune liver diseases. World J Gastroenterol. 2008;14:3388-3395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Freeman RB, Gish RG, Harper A, Davis GL, Vierling J, Lieblein L, Klintmalm G, Blazek J, Hunter R, Punch J. Model for end-stage liver disease (MELD) exception guidelines: results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transpl. 2006;12:S128-S136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 138. | Abraham SC, Kamath PS, Eghtesad B, Demetris AJ, Krasinskas AM. Liver transplantation in precirrhotic biliary tract disease: Portal hypertension is frequently associated with nodular regenerative hyperplasia and obliterative portal venopathy. Am J Surg Pathol. 2006;30:1454-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 139. | Milkiewicz P, Wunsch E, Elias E. Liver transplantation in chronic cholestatic conditions. Front Biosci (Landmark Ed). 2012;17:959-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 140. | Neuberger J. Liver transplantation for primary biliary cirrhosis: indications and risk of recurrence. J Hepatol. 2003;39:142-148. [PubMed] [Cited in This Article: ] |
| 141. | Garcia CE, Garcia RF, Gunson B, Christensen E, Neuberger J, McMaster P, Mirza DF. Analysis of marginal donor parameters in liver transplantation for primary biliary cirrhosis. Exp Clin Transplant. 2004;2:183-188. [PubMed] [Cited in This Article: ] |
| 142. | Kashyap R, Safadjou S, Chen R, Mantry P, Sharma R, Patil V, Maloo M, Ryan C, Marroquin C, Barry C. Living donor and deceased donor liver transplantation for autoimmune and cholestatic liver diseases--an analysis of the UNOS database. J Gastrointest Surg. 2010;14:1362-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 143. | Wiesner RH. Liver transplantation for primary sclerosing cholangitis: timing, outcome, impact of inflammatory bowel disease and recurrence of disease. Best Pract Res Clin Gastroenterol. 2001;15:667-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 144. | Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 145. | Leidenius M, Höckersted K, Broomé U, Ericzon BG, Friman S, Olausson M, Schrumpf E. Hepatobiliary carcinoma in primary sclerosing cholangitis: a case control study. J Hepatol. 2001;34:792-798. [PubMed] [Cited in This Article: ] |
| 146. | Goss JA, Shackleton CR, Farmer DG, Arnaout WS, Seu P, Markowitz JS, Martin P, Stribling RJ, Goldstein LI, Busuttil RW. Orthotopic liver transplantation for primary sclerosing cholangitis. A 12-year single center experience. Ann Surg. 1997;225:472-481; discussion 481-483. [PubMed] [Cited in This Article: ] |
| 147. | Graziadei IW, Wiesner RH, Marotta PJ, Porayko MK, Hay JE, Charlton MR, Poterucha JJ, Rosen CB, Gores GJ, LaRusso NF. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 148. | Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 331] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 149. | Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820-833. [PubMed] [Cited in This Article: ] |
| 150. | Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1:735-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 295] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 151. | Kogan J, Safadi R, Ashur Y, Shouval D, Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J Clin Gastroenterol. 2002;35:75-81. [PubMed] [Cited in This Article: ] |
| 152. | Reich DJ, Fiel I, Guarrera JV, Emre S, Guy SR, Schwartz ME, Miller CM, Sheiner PA. Liver transplantation for autoimmune hepatitis. Hepatology. 2000;32:693-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 153. | Liberal R, Longhi MS, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis after liver transplantation. Clin Gastroenterol Hepatol. 2012;10:346-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 154. | Futagawa Y, Terasaki PI. An analysis of the OPTN/UNOS Liver Transplant Registry. Clin Transpl. 2004;315-329. [PubMed] [Cited in This Article: ] |