Copyright
©The Author(s) 2015.
World J Hepatol. May 28, 2015; 7(9): 1287-1296
Published online May 28, 2015. doi: 10.4254/wjh.v7.i9.1287
Published online May 28, 2015. doi: 10.4254/wjh.v7.i9.1287
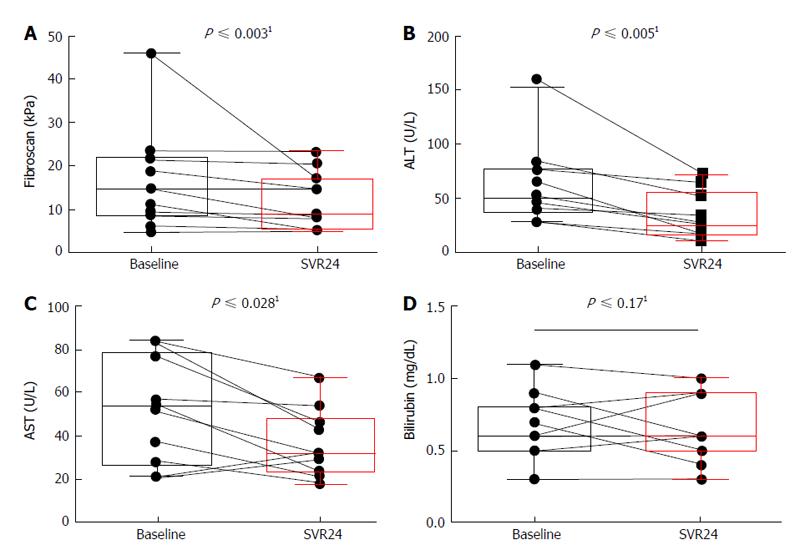
Figure 1 Development of liver parameters during treatment.
A: Liver stiffness in correlation to fibrosis was determined by fibroscan at baseline and end of treatment. Measurements are calculated in kPa. Student’s t-test was used to compare categorical characteristics, aP-value of < 0.01 was considered to be significant; ALT (B), AST (C) and bilirubin (D) were determined at baseline and end of treatment. Student’s t-test was used to compare categorical characteristics, aP-value of < 0.01 was considered to be significant. 1Wilcoxon signed-rank-test, P≤ 0.05 set as statistic significant. SVR: Sustained viral response; ALT: Alanine aminotransferases; AST: Aspartate aminotransferase.
- Citation: Herzer K, Papadopoulos-Köhn A, Achterfeld A, Canbay A, Piras-Straub K, Paul A, Walker A, Timm J, Gerken G. Management of telaprevir-based triple therapy for hepatitis C virus recurrence post liver transplant. World J Hepatol 2015; 7(9): 1287-1296
- URL: https://www.wjgnet.com/1948-5182/full/v7/i9/1287.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i9.1287