INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease and is a major public health problem worldwide[1-3]. It is defined as accumulation of lipid deposits in the hepatocytes that are not due to excessive alcohol use[4]. NAFLD encompasses a spectrum of diseases ranging from simple fatty liver (hepatosteatosis) to nonalcoholic steatohepatitis (NASH), which in its most severe form can lead to liver fibrosis, cirrhosis and hepatocellular carcinoma[3,5-7].
The pathophysiology of NAFLD has still not been exactly clarified. In 1998, Day et al[8] put forward the widely known “two-hit” hypothesis. The ‘‘two-hit” hypothesis is the commonly accepted model to explain the development of NAFLD and the progression from simple steatosis to NASH. The ‘‘first hit’’ is the collection of lipids in the hepatocytes and insulin resistance is the key pathogenic factor for the development of hepatosteatosis. The ‘‘second hit’’ leads to inflammation, hepatocyte injury and fibrosis. Oxidative stress, adipokines, proinflammatory cytokines and mitochondrial dysfunction are factors that induce the second hit[5,8,9]. However, there is growing evidence that this hypothesis is likely incorrect. It has been shown that simple steatosis and NASH are two distinct entities with different pathogenetic pathways. Nowadays, one of the accepted theories is “multiple parallel hits”. The initial hypothesis was based on insulin resistance causing increased uptake and synthesis of free fatty acids; on the other hand, “multiple parallel hits” theory includes oxidative stress from reactive oxygen species and varying production of adipokines which plays a major role in the pathogenesis of NASH. Another theory for explaining the progression from NAFLD to NASH is named “distinct-hit” pathogenetic heterogeneity obtained via at least two different ways. Genetic predisposition and timing seem to lead to activation of different ways which causes simple steatosis and NASH[10].
The prevalence of NAFLD has been reported to be 10%-46% in the United States and 6%-35% in the rest of the world[11]. With the increasing prevalence of obesity, type 2 diabetes mellitus and metabolic syndrome, there is a dramatic increase in the frequency of NAFLD. The prevalence of NAFLD in children and adolescents is also increasing. The increasing prevalence has resulted in an increasing need for NASH-related liver transplantation in the last 10 years[12]. Therefore, early diagnosis and treatment is quite important.
The diagnosis of NAFLD requires evidence of fatty infiltration of the liver in the absence of excessive alcohol consumption and other secondary causes of chronic liver disease. According to all recent guidelines, liver biopsy is still the best standard for diagnosis and staging of NAFLD. It is also a reliable method for differentiating NASH from simple steatosis[3,4,11]. However, biopsy is an invasive and impractical method for assessment of at risk patients with NAFLD due to the high disease prevalence. It is highly dependent on the experience of the operator and major complications occur in 0.1%-2.3% of cases[11]. Furthermore, this method is unsuitable for screening and follow-up of patients with NAFLD. If biopsy samples are small in size, they are subject to sampling error and interobserver variability[13,14]. Nonexpert physicians and patients are waiting for an almost perfect noninvasive test which is a biomarker with less than 10% of false positive or false negative results and more than 99% applicability. Therefore, it is an illusion to wait for an almost perfect biomarker with an adjusted area under the receiver operator curve greater than 90% for the diagnosis of NASH. For this reason, noninvasive and simple imaging methods came into use in the diagnosis and evaluation of NAFLD, such as ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) with chemical shift imaging (CSI) and magnetic resonance spectroscopy (MRS) and elastography with US and MRI. This article will review the importance of these imaging methods and recent developments in the diagnosis of NAFLD.
IMAGING MODALITIES
US
US is the primary imaging method used to determine and identify the fatty liver[15]. US is widely used for screening asymptomatic patients with increased liver enzymes and suspected NAFLD. It is safe, non-invasive, non-radiation, widely available, cost effective and an accurate tool in the detection of fatty liver[16]. The convex probe (2-5 MHz) can be used in the examination. Right kidney echogenicity is used for the identification of liver parenchyma echogenicity. Nonsteatotic liver parenchyma shows homogeneous echo texture with similar or a bit higher echogenicity when compared to the kidney cortex and spleen parenchyma. Fatty liver shows echogenicity (bright liver) greater than the kidney cortex and spleen parenchyma due to intracellular accumulation of fat vacuoles[3,15,17]. In addition, US findings of fatty liver include hepatomegaly and vascular blurring of the portal or hepatic vein[4].
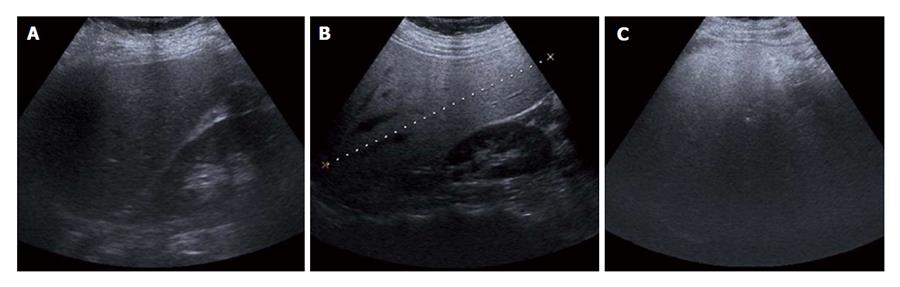
The grades of fatty liver (hepatosteatosis) described previously at US are qualitatively defined using a four-point scale as follows: normal, mild, moderate or severe[14,17-20]. With the same kidney cortex and liver parenchyma echogenicity, it is evaluated as: normal, no fatty liver (grade 0); mild (grade 1; Figure 1A), mildly diffuse increase in liver echogenicity and clear visualization of the diaphragm and intrahepatic vessel walls; moderate (grade 2; Figure 1B), moderate grade diffuse increase in liver echogenicity obscuring the intrahepatic vessel walls and the diaphragm; severe (grade 3; Figure 1C), prominent liver echogenicity increment in liver echogenicity and poor or nonvisualization of the hepatic vessels and diaphragm.
Figure 1 Ultrasonographic images show the hepatosteatosis stages.
A: Grade 1: mild fatty liver; B: Grade 2: moderate fatty liver; C: Grade 3: severe fatty liver.
US is often useful for characterization of grade 2 or grade 3 hepatosteatosis but less effective for diagnosing grade 1 hepatosteatosis. Furthermore, it is difficult to distinguish liver fibrosis from hepatosteatosis[17,18,21]. In studies, the sensitivity and specificity of US in detecting hepatosteatosis have been found to be 60%-94% and 84%-95%, respectively[16,18,22,23]. Hamaguchi et al[24] reported that US has a high sensitivity (91.7%) and specificity (100%) for fatty liver detection. Palmentieri et al[25] reported the finding of 235 patients undergoing US with liver biopsy and found the sensitivity, specificity, positive predictive value and negative predictive value to be 91%, 93%, 89% and 94%, respectively, for calculating at least 30% steatosis.
Hepatorenal sonographic index is known as the ratio between the mean brightness level of the right kidney and the liver and has also been suggested as a measure of hepatosteatosis. A study found very high sensitivity (100%) and specificity (91%) with a cut-off of 1.49 for the diagnosis of hepatosteatosis > 5%[26].
Quantitative methods of measuring liver echogenicity are always unreliable[27,28] but quantitative calculation of hepatosteatosis is more accurate than the qualitative assessment of hepatosteatosis on US. The ratios of the quantitative assessment were 77%, 77% and 71% as the sensitivity, specificity and diagnostic accuracy, respectively, in comparison with 60%-100%, 77%-95% and 96% for qualitative assessment[15,17,28].
Despite the benefits of US, such as being non-invasive, widely available, low cost, ease of clinician use and interpretation, it has some limitations, such as a small field of view, limited use in accompanying chronic liver disease, inability to distinguish degree of fibrosis, cirrhosis and NASH, operator and equipment dependence, limited use in obese patients and low sensitivity when hepatosteatosis is less than 20%-30%[15,29]. In a recent study, Iijima et al[30] used an US contrast matter (Levovist; Sherling, Berlin) to distinguish between simple hepatosteatosis and NASH. They found a significant decrease in the uptake of Levovist associated with fibrosis in NASH patients. Further clinical and technical investigations are needed to overcome the limitations of US.
CT
CT evaluation of hepatosteatosis is dependent on the attenuation values, called Hounsfield units (HUs), of the liver parenchyma[3]. The best CT method for the calculation of fatty liver is unenhanced CT which allows for a more quantitative evaluation of liver attenuation[4,31]. Based on the physical characteristics of X-ray penetration of tissue, the attenuation in unenhanced CT is measured. The degree of decrease in attenuation on unenhanced CT is the most decisive of the degree of liver fat content[31]. Due to the attenuation characteristics that are based on various factors regarding to the contrast material and scan timing, unenhanced CT is more commonly used than enhanced CT[3,15,32].
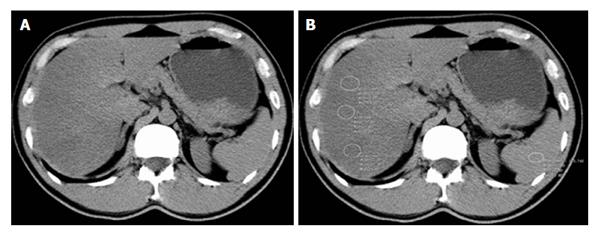
Unenhanced CT can be especially used for evaluating the fatty liver in a transplant donor. It has an important place in diagnosing hepatosteatosis of ≥ 30%, with 100% specificity and 82% sensitivity[15,33]. Three techniques are used to evaluate fatty liver with CT: the absolute measurement of attenuation values (in HUs); the difference in attenuation values between liver and spleen; and the ratio of these values of the liver attenuation index[31,33,34]. Normal liver has an attenuation value of about 50-65 HU, which is about 8-10 HU higher than a normal spleen[15]. If the liver attenuation is less than 48 HU, fatty liver infiltration is diagnosed[35]. With unenhanced CT, liver attenuation values less than 40 HU or a liver-to-spleen attenuation difference > 10 HU is highly predictive of hepatosteatosis[16,36] (Figure 2). Kodama et al[31] reported that 40 HU liver attenuation shows fatty infiltration of about 30%. They found that attenuation values of liver CT of 64.4 HU, 59.1 HU, 41.9 HU and 25.0 HU at unenhanced scanning correlated with the fatty infiltration degrees of 0%, 1%-25%, 26%-50% and more than 50%. Furthermore, a liver-to-spleen ratio of less than 1 is sometimes used to diagnose fatty liver infiltration[34]. Park et al[33] reported that a liver-to-spleen attenuation ratio of < 0.8 and the liver-to-spleen attenuation difference less than -9 HU has a high specificity (100%) for the diagnosis of grade 2 to 3 hepatosteatosis[16]. However, the sensitivity of the two measures (liver-to-spleen attenuation ratio and liver-to-spleen attenuation difference) for the diagnosis of grade 2-3 macrovesicular hepatosteatosis of more than 30% is between 73%-82%[15,33,37].
Figure 2 Computed tomography evaluation of fatty liver using a liver-to-spleen attenuation difference with unenhanced computed tomography.
A: Diffuse fatty infiltration of liver with attenuation much lower than the spleen on visual analysis; B: Multiple regions-of-interest (white circles, ROIs) show mean hepatic attenuation (25 HU) and splenic attenuation (51 HU) with -26 HU liver-to-spleen attenuation difference, pointing to moderate-to-severe hepatosteatosis.
Dual energy CT has great potential and quite a few conceivable clinical indications. It can differentiate between several chemical components in tissue and also be used to quantify fatty liver and includes acquisition at two tube potentials with 80-140 kVp. The theoretical advantages of it have been unsettled clinically until now. There is a decline in CT liver attenuation at low energy level in hepatosteatosis. When the tube potential increases, the fat attenuation increases. Studies have reported that an attenuation alteration of > 10 HU with the increment of the tube potential from 80 to 140 kVp is considered to have fatty liver infiltration of > 25%[16,38].
Although CT is a quick, non-operator dependent imaging method, radiation exposure should be always kept in mind. CT was quite accurate for the diagnosis of grade 2-3 steatosis but was not as accurate for detecting grade 1 steatosis. In addition, liver parenchymal attenuation in CT may be affected by some factors, including the presence of excess iron and glycogen in the liver and the certain drugs such as amiodarone and methotrexate, acute hepatitis or acute toxic hepatic injury and cirrhosis[15,39,40]. Therefore, in patients with hemochromatosis and hemosiderosis, liver attenuation values are unreliable for detecting fat infiltration[37].
MRI
MRI is one of the most sensitive imaging methods for detection and characterization of fatty liver. It is a radiation-free modality to detect fatty liver, even in microscopic quantities. The degree of fatty infiltration can be calculated with CSI or MRS. A good correlation has been found between MRI and histology in patients with NAFLD. It may detect steatosis at a level as low as 3%[41]. The principal MRI physics used in both techniques to differentiate protons in fat from those in water is the chemical shift phenomenon.
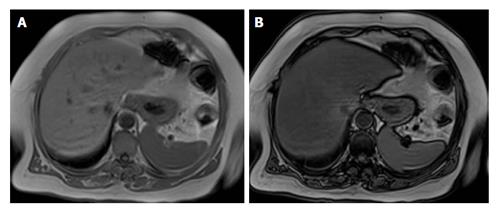
Chemical shift imaging is a method commonly used because of its easy applicability and high accuracy. Chemical shift techniques are caused by the difference between the mobility frequencies of fat and water protons in order to accurately detect and quantify fatty infiltration[42,43]. The said frequency difference produces tissues that contain fat and water in order to lose signal intensity when the proton magnetizations are opposed in out-of-phase imaging. The normal liver parenchyma shows similar signal intensity on in-phase (IP) and out-of-phase (OP) images. The loss in signal intensity can be observed when out-of-phase images are compared to the in-phase images (Figure 3). Whereas the normal liver parenchyma shows similar signal intensity on in-phase and out-of-phase images, fatty liver exhibits decreased signal intensity on out-of-phase images in the presence of severe fatty infiltration[43].
Figure 3 Magnetic resonance imaging evaluation of fatty liver using chemical shift imaging.
A: In-phase image; B: Out-of-phase image. When out-of-phase image is compared with in-phase images, it shows the signal intensity decrease.
On the 1.5 Tesla MRI, the frequency shift between fat and water is approximately 220 Hz, which results in OP phase condition at a TE of about 2.4 ms and IP condition at a TE of about 4.8 ms. With the introduction of 3 Tesla MRI, the evaluation of fatty liver has increased. The chemical shift difference between fat and water at 3 Tesla is about 415 Hz[15,44]. With this frequency difference, both İP and OP images can be obtained in a single breath hold by helping to avoid motion artifacts.
Magnetic resonance spectroscopy is one of the most correct imaging methods for noninvasive evaluation of fatty liver[45]. Single-voxel MRS gives significant information regarding the chemical composition of the normal organ and chemical changes in the fatty liver such as NAFLD. Small fat amounts can be quantified by this method. In addition, it is particularly useful in some cases, such as the elimination of liver biopsy necessity during the presurgical assessment of liver transplant donors and evaluation of the response to treatment of longitudinal follow-up of patients with metabolic disorders or obesity.
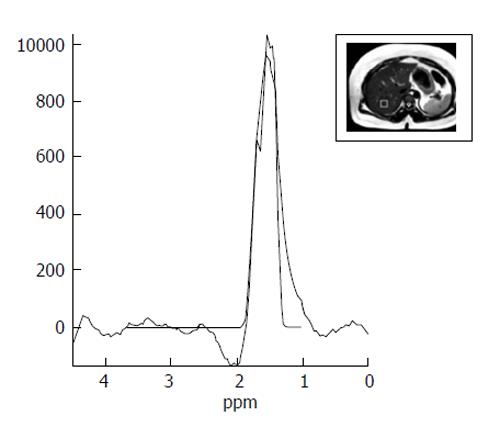
MRS evaluates proton signals as a function of their resonant frequency and shows multiple peaks at different locations (Figure 4). On MRS spectra of the liver, most of the visible peaks are produced from water and fat. The water occurs as a single peak at 4.7 ppm and fat occurs as multiple peaks due to the presence of various chemical components in fat (e.g., at 1.3 ppm a methylene (CH2) peak and other smaller peaks at different locations)[3]. The values obtained with MRS display show a good correlation with the results of liver biopsy. Hence, it is proposed as an optimal imaging method for calculating the content of hepatic triglyceride[46].
Figure 4 Magnetic resonance spectroscopy image shows a lipid peak in a case of grade 3 hepatosteatosis.
Technically, either a stimulated echo acquisition mode (STEAM) or a point-resolved spectroscopy (PRESS) sequence can be used. PRESS sequences provide a higher signal-to-noise ratio than STEAM sequences. However, STEAM is believed more suitable for fat quantification as it is less sensitive to a J-coupling effect[3,47]. MRS sequences should be optimized to minimize relaxation effects. A long repetition time (TR), typically longer than 3000 ms at 1.5 Tesla MRI, can minimize T1-relaxation effects. T2-relaxation effects can be decreased by using the shortest possible echo times (TE).
In evaluating fatty liver, apart from CSI and MRS, other methods such as fat saturation and fat-selective excitation approaches can be used[42,48,49]. The signal intensity loss of liver on T2-weighted fat-saturated rapid SE images in comparison with T2-weighted non-fat-saturated rapid SE images is indicative of fatty infiltration.
The MRI sensitivities and specificities in detecting histological steatosis ≥ 5% were 76.7%-90.0% and 87.1%-91%, respectively, and the MRS performances were 80%-91% and 80.2%-87%, respectively[50,51]. MRI with CSI and MRS have a higher diagnostic accuracy than US or CT and these methods can evaluate hepatosteatosis in an objective manner using the quantitative index.
MRI with CSI have several advantages over MRS. The acquisition and analysis of MRS information requires expertise and is time consuming and complex. Because single-voxel MRS accumulates information from a small portion of the liver it may cause a sampling error. By comparison, MRI is easily applicable, commonly available and it may evaluate the entire liver within a short breath hold[7].
Elastography
Although imaging methods such as US, CT and MRI can evaluate hepatosteatosis, none of them can evaluate liver fibrosis and NASH[11,52]. Noninvasive evaluation of liver fibrosis and NASH can be mainly performed by US elastography and MR elastography. Both techniques evaluate liver stiffness by measuring the velocity of shear wave using US or MRI. Several US elastography techniques have been defined. These includes transient elastography, supersonic shear wave elastography, acoustic radiation force impulse elastography (ARFI) and real-time tissue elastography.
Transient elastography (FibroScan) is performed with pulse-echo US and measures liver stiffness as a function of the extent of liver infiltration. It can detect liver cirrhosis with high accuracy but the accuracy is decreased at lower fibrosis stages[53,54]. Studies have reported highly accurate rates in distinguishing severe liver fibrosis from mild liver fibrosis, with 88.9%-100% sensitivities and 75%-100% specificities[54-57]. In a study of 246 NAFLD patients, using US elastography for the diagnosis of moderate fibrosis, bridging fibrosis and cirrhosis were found to be 0.84, 0.93 and 0.95, respectively[58]. Controlled attenuation parameter (CAP) has been proposed as a noninvasive method for the determination and measurement of hepatic steatosis. The mechanism of CAP is the reduction in amplitude of ultrasound that can be estimated as it is amplified through the liver tissue using the same radio-frequency data used for estimation of liver stiffness using Fibroscan (Echosens, Paris, France), an ultrasound based vibration-controlled transient elastography device[59]. The shear stiffness of normal liver is between 6.5 and 7 kPa. ARFI is also performed in a similar form and measures shearing velocity. Normal velocity of the liver is 1 m/s. This velocity is reduced when there is fatty infiltration[16]. The other alternative methods to transient elastography are rarely used currently.
MR elastography appears to be superior to transient elastography in evaluating liver fibrosis. It evaluates larger liver volumes and is unaffected by obesity[60]. However, data are so far limited in NAFLD patients. Furthermore, its low availability and high cost limits its use in clinical practice and more studies of MR elastography are needed.
In conclusion, imaging methods allow both qualitative and quantitative evaluation of fatty liver. US is a safe, relatively cheap, easily accessible technique with no contraindications for screening of NAFLD. Even so, limited sensitivity for mild steatosis, operator dependency, patient factors (gas and obesity) are the main disadvantages. CT has excellent specificity but low sensitivity for mild hepatic steatosis. Especially for the longitudinal follow-up of patients, radiation exposure is the main disadvantage of CT. MRS is currently the most accurate imaging method used to diagnose hepatosteatosis. Technical optimization of MRS and MRI with CSI may result in a highly accurate diagnostic rate and these methods may replace the liver biopsy as the reference standard for research investigations. US elastography and MR elastography can diagnose liver fibrosis associated with NAFLD and may play a role in the characterization of NASH. However, further studies are needed to increase the sensitivity and specificity of imaging methods in the diagnosis of hepatosteatosis and steatohepatitis.