Published online Nov 28, 2015. doi: 10.4254/wjh.v7.i27.2774
Peer-review started: September 19, 2015
First decision: October 21, 2015
Revised: November 2, 2015
Accepted: November 10, 2015
Article in press: November 11, 2015
Published online: November 28, 2015
Processing time: 70 Days and 5.9 Hours
AIM: To determine the clinical impact of portal vein thrombosis in terms of both mortality and hepatic decompensations (variceal hemorrhage, ascites, portosystemic encephalopathy) in adult patients with cirrhosis.
METHODS: We identified original articles reported through February 2015 in MEDLINE, Scopus, Science Citation Index, AMED, the Cochrane Library, and relevant examples available in the grey literature. Two independent reviewers screened all citations for inclusion criteria and extracted summary data. Random effects odds ratios were calculated to obtain aggregate estimates of effect size across included studies, with 95%CI.
RESULTS: A total of 226 citations were identified and reviewed, and 3 studies with 2436 participants were included in the meta-analysis of summary effect. Patients with portal vein thrombosis had an increased risk of mortality (OR = 1.62, 95%CI: 1.11-2.36, P = 0.01). Portal vein thrombosis was associated with an increased risk of ascites (OR = 2.52, 95%CI: 1.63-3.89, P < 0.001). There was insufficient data available to determine the pooled effect on other markers of decompensation including gastroesophageal variceal bleeding or hepatic encephalopathy.
CONCLUSION: Portal vein thrombosis appears to increase mortality and ascites, however, the relatively small number of included studies limits more generalizable conclusions. More trials with a direct comparison group are needed.
Core tip: Portal vein thrombosis (PVT) is a common complication of cirrhosis with resultant downstream hepatic decompensation and mortality. Treatment options carry risk and are not without complications. To date, there is a lack of systematic evidence on the clinical importance of PVT. We performed a systematic review and meta-analysis to determine the aggregate estimates of effect of PVT on hepatic decompensation and mortality. PVT appears to significantly increase mortality (OR = 1.62, 95%CI: 1.11-2.36) and ascites (OR = 2.52, 95%CI: 1.63-3.89), however, the small number of included studies limits more generalizable conclusions. More trials with a direct comparison group are needed.
- Citation: Stine JG, Shah PM, Cornella SL, Rudnick SR, Ghabril MS, Stukenborg GJ, Northup PG. Portal vein thrombosis, mortality and hepatic decompensation in patients with cirrhosis: A meta-analysis. World J Hepatol 2015; 7(27): 2774-2780
- URL: https://www.wjgnet.com/1948-5182/full/v7/i27/2774.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i27.2774
Portal vein thrombosis (PVT) is defined as an obstruction of the portal vein or its branches, which include the splenic, superior mesenteric, and inferior mesenteric veins[1]. It is associated with numerous conditions including malignancy, myeloproliferative disorders, inflammatory conditions (such as pancreatitis), intra-abdominal infections (such as secondary peritonitis), and cirrhosis[2,3]. PVT is common in patients with cirrhosis; over 30% of liver transplant recipients have PVT on direct explant examination at the time of transplant (LT)[2,4,5]. Incidence rates of PVT while variable, are reported to be as high as 16%[6]. The mechanism of PVT development in cirrhosis is multifactorial and is due to a combination of changes in liver architecture leading to impaired blood flow and endothelial cell activation, hypercoagulability, and the potential development of hepatocellular carcinoma (HCC)[7]. The presence of PVT appears to be associated with the severity of underlying liver disease and hepatic decompensation from a mechanistic standpoint. However, the field of coagulation disorders and chronic liver disease is ever evolving and continues to generate controversy, in particular, when consideration is given to the impact of PVT on the development of hepatic decompensation. Multiple studies have been published indicating adverse clinical outcomes in the setting of PVT in both transplant and non-transplant populations, including hepatic decompensation, increased post-transplant mortality, and decreased quality of life[7-10]. Others have argued that PVT does not affect clinically relevant outcomes[11]. Due to this uncertainty, we sought to determine the clinical impact of PVT on transplant free survival and hepatic decompensation in adult patients with cirrhosis.
The investigators systematically searched the published medical literature for observational studies and clinical trials that compared mortality or hepatic decompensation outcomes in cirrhosis patients with and without PVT. Published studies were identified by searching the following electronic databases: MEDLINE, Scopus, Science Citation Index, AMED, and the Cochrane Library. The search criteria included all publications through February 2015 with English language restriction. Electronic search criteria included the following terms or keywords: “portal vein thrombosis”, “mesenteric thrombosis”, “splanchnic thrombosis”, “cirrhosis”, “mortality”, “decompensation”, and “humans”. We reviewed the reference lists of included articles in order to identify articles missed in the database searches. Recent conference abstract lists and other relevant grey literature sources were also searched for examples of relevant studies using the same terms and keywords. Studies were excluded if PVT was associated with only malignancy, developed post-procedure (surgery or interventional), were found in non-cirrhotic patients with portal hypertension, were in LT-only recipients, if no control/comparison group without PVT was included, or if survival was not analyzed. This study did not require institutional review board approval.
Two study personnel (Stine JG and Shah PM) independently screened the abstracts and titles of all studies identified using the electronic and manual search criteria to identify studies meeting the inclusion criteria. Each study meeting requirements of the first-round inclusion criteria then underwent a full-text independent review by both reviewers. Disagreements about inclusion between reviewers were resolved by follow-up consultation, and if necessary by a third clinical reviewer (Cornella CL). Two reviewers independently extracted the following data from each study that met inclusion criteria: patient characteristics (age, gender, MELD, and etiology of liver disease), study-level characteristics (author, publication year, study design, enrollment period, target population, total number of enrolled patents, and percentage of patients with PVT) and outcomes (mortality and hepatic decompensation).
Mortality was the primary outcome assessed. Secondary outcomes included the presence of or development of new gastroesophageal variceal bleeding, ascites, hepatic encephalopathy, and an aggregate measure of the occurrence of any of these three hepatic decompensation outcomes.
The quality of observational studies was assessed using the methods described by Stroup et al[12]. Only studies deemed high-quality by the investigators were included in the analysis. The Newcastle-Ottowa Quality Assessment for Cohort Studies scale[13] was used to further characterize the quality of studies based on selection of study groups, comparability of groups, and ascertainment of outcome. The studies are rated on an 8-point scale separated by the three broad sections delineated above.
Descriptive analysis of the studies identified, excluded, and included, and meta-analysis of the reported study effect measures, was conducted utilizing review manager software (Rev-Man version 5.3; Copenhagen; The Nordic Cochrane Centre; The Cochrane Collaboration; 2014). We estimated pooled ORs and calculated corresponding 95%CIs using DerSimonian and Laird random-effects models, which account for both between and within study variability given that the included studies were not functionally identical[14,15]. Between study variability was separately assessed using the Cochran’s Q statistic (with P < 0.05 considered significant). The proportion of heterogeneity accounted for by between-study variability was estimated using the I2 index and adjudicated to be significant if I2 was > 75%[14,15]. A post-hoc funnel plot was created to assess for the presence or absence of publication bias.
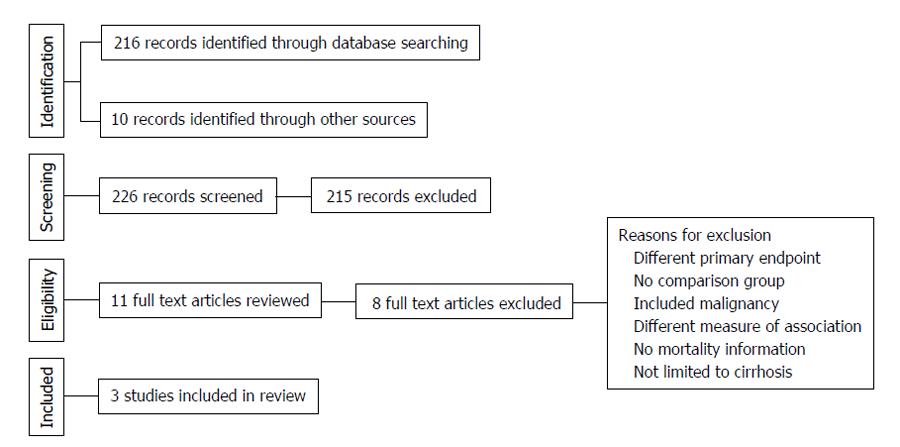
The electronic search criteria identified 226 studies. After ensuring no duplicates were present, we screened titles and abstracts. The full text of eleven studies was assessed for eligibility. Following the qualitative systematic review process, three observational studies met the inclusion criteria for the current meta-analysis[7,16,17]. Of these, two were retrospective[7,17]. The third study followed a prospective cohort of patients[16]. No additional studies were appropriate for inclusion based on our a-priori determined inclusion and exclusion criteria. Nery et al[11] recently published a multicenter prospective series of 1243 adult patients with cirrhosis without baseline PVT in France and Belgium. About 118 patients developed de novo PVT during a median follow-up period of 47 mo. This study, while initially considered in full-text review, was excluded specifically because absolute numbers for mortality or individual types of hepatic decompensation were not provided; rather, univariate and multivariable analysis P-values were provided only and only a composite of hepatic decompensation was given in absolute number.
The 3 eligible reports evaluated cirrhotic patients that did not initially have PVT, but developed it sometime over the study period. They each excluded patients with HCC and prior transplant. All 3 studies evaluated long-term outcomes in cirrhotic patients with PVT compared to cirrhotic patients without PVT. Study level characteristics are found in Table 1. A summary of the search results is presented in Figure 1, reflecting the reporting standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[18].
The Englesbe et al[7] study assessed a total of 3295 (148 with PVT and 3147 without PVT) cirrhotic patients between 1995-2007. The study assessed patients that were being evaluated as candidates for liver transplantation that had thrombus in the main portal vein only. Patients with partial thrombus or thrombi in portal vein branches, without extension into the main portal vein were excluded. The Maruyama et al[17] article evaluated a total of 150 patients with viral hepatitis, 42 had PVT (and 108 did not have PVT). The study by John et al[16] found 290 patients with cirrhosis, 70 of these had PVT and 220 did not. Notably, both the Maruyama et al[17] and John et al[16] articles specified patients with complete and partial thrombus, unlike the Englesbe study[7].
In total, the three studies included 3735 cirrhotic patients, 260 of which had PVT. Lengths of follow-up ranged from less than one month to 136 mo, with mean follow-ups ranging from 25 mo to 50 mo. Baseline demographic characteristics were similar between PVT and non-PVT groups in all 3 studies. There were no differences in regards to race, age, gender, causes of cirrhosis, or model for end stage liver disease (MELD) scores. Demographic and etiologic characteristics of the patients included in each studies are summarized in Table 2.
| Englesbe 20101 | John 20132 | Maruyama 20133 | ||||
| No PVT (n = 3147) | PVT (n = 148) | No PVT (n = 220) | PVT (n = 70) | No PVT (n = 108) | PVT (n = 42) | |
| Sex (M/F) | 1905/1242 | 91/57 | 144/76 | 42/28 | 63/45 | 22/20 |
| Race | ||||||
| Black | 218 (6.9) | 7 (4.7) | - | - | - | - |
| White | - | - | 184 (83.6) | 60 (85.7) | - | - |
| Other | 2545 (80.9) | 131 (88.5) | - | - | ||
| "Nonblack" | "Nonblack" | - | - | |||
| Etiology | ||||||
| AIH | 95 (3) | 8 (5.4) | - | - | - | - |
| Biliary/cholestatic | 173 (5.4) | 7 (5.4) | 24 (11) | 5 (7.1) | - | - |
| Alcohol | 672 (21.4) | 31 (20.9) | 37 (16.9) | 5 (7.1) | - | - |
| Viral | 1253 (39.8) | 50 (34.1) | 62 (28.3) | 16 (27.1) | 108 | 42 |
| Cryptogenic/NASH | 72 (2.3) | 0 | 35 (16) | 12 (17.1) | - | - |
| Other | 449 (14.3) | 22 (14.9) | 37 (16.9) | 20 (28.6) | - | - |
| Age (yr) | 51.5 ± 11.2 | 50.9 ± 10.8 | 55.8 ± 9.1 | 58.4 ± 8.8 | 63.3 ± 8.68 | 62.4 ± 11 |
| MELD | 12.1 ± 7.2 | 13.3 ± 8.3 | 13.8 ± 4.5 | 14.9 ± 5.9 | 10.2 | 10.6 |
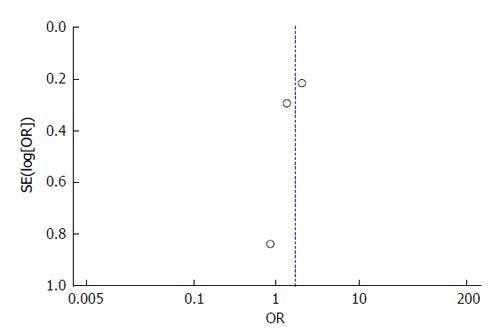
All 3 studies include cohorts drawn from their abdominal transplant clinic population. The patients were truly representative of the average transplant population. Exposed and unexposed patients (patients with cirrhosis with spontaneous PVT and those without PVT) were all drawn from their transplant clinic population and all information was obtained through medical records in the three studies. The Englesbe et al[7] study controlled for PVT along with MELD, age, and presence of hepatitis C virus in a multivariable logistic regression with survival as the outcome. The Maruyama et al[17] study does not include a model controlling for covariates. The John et al[16] study does control for ascites and renal function, however this study created a multivariable model to identify predictors for PVT development. All three studies had widely variable amounts of follow-up and there is no information available on patients lost to follow-up for any of the studies. Based on these characteristics, the Englesbe et al[7] study received a score of 7/8, while Maruyama et al[17] and John et al[16] both received 6/8. The potential for publication bias was assessed using a funnel plot of the relationship between reported effect variance SE(log[OR]) and the reported study OR. The plot illustrates the lack of evidence for potential publication bias in the three studies-the study with the largest effect size is the largest study included while the smaller studies have lower effect sizes (Figure 2).
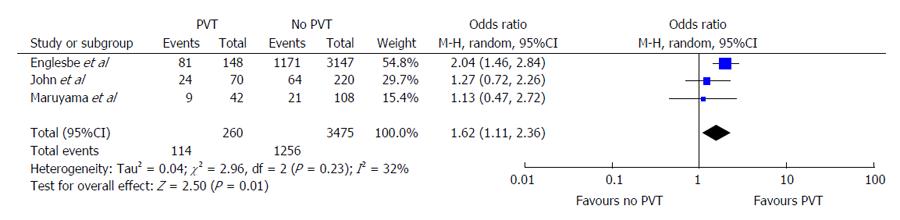
The Englesbe et al[7] article demonstrated an incidence rate of 4.5% for PVT. The patients with PVT were at significantly higher risk of mortality with an OR of 2.04 (95%CI: 1.46-2.84) (Figure 3) Conversely, the Maruyama et al[17] and John et al[16] studies found higher rates of mortality between PVT and non-PVT subjects. The OR for mortality for PVT in both studies were 1.13 and 1.27, respectively but these differences were non-significant. Pooled analysis of the results reported across all 3 studies demonstrates a significantly increased risk of mortality in PVT patients (OR = 1.62, 95%CI: 1.11-2.36, P = 0.01). The Cochran’s Q statistic was non-significant at P = 0.23 and I2 = 32%, demonstrating non-significant heterogeneity of effects reported across studies.
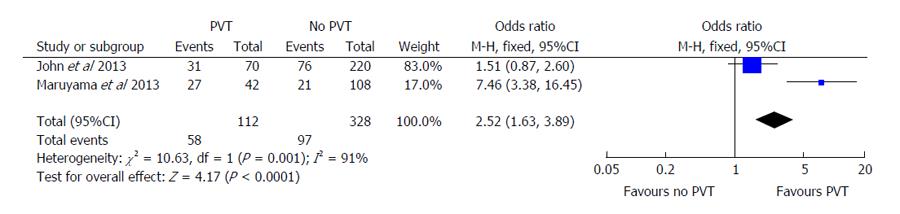
Secondary outcomes included episodes of hepatic decompensation, including individual cases of ascites, variceal bleeding, or portosystemic encephalopathy. Both John et al[16] and Maruyama et al[17] demonstrated similar effects of PVT on ascites development. John et al[16] showed an OR of 1.51 (95%CI: 0.87-2.60) compared to an OR of 7.46 (95%CI: 3.38-16.45) in the Maruyama et al[17] study (Figure 4). The Englesbe et al[7] study was excluded from this portion of analysis since they did not report on rates of hepatic decompensation. When the pooled OR was evaluated using random-effects modeling, PVT continued to have statistically significant higher odds of hepatic decompensation (OR = 2.52, 95%CI: 1.63-3.89, P < 0.001). The Cochran’s Q statistic and I2 were both significant for heterogeneity in this analysis. There was insufficient data available to determine the pooled effect on other markers of decompensation including gastroesophageal variceal bleeding or hepatic encephalopathy.
Our systematic review and meta-analysis is the first study to offer a pooled estimate and quantitative assessment of the clinical impact of PVT in terms of both mortality and hepatic decompensation. We have demonstrated a significantly increased rate of mortality for patients with cirrhosis and PVT when compared to those patients without PVT. This finding is important because PVT is a common finding in patients with cirrhosis[4,6,19] and one that significantly impairs quality of life and post-LT outcomes[7-9]. Several risk factors have been suggested to increase the risk of PVT in patients awaiting liver transplantation, including non-alcoholic steatohepatitis[20].
The risk of hepatic decompensation with ascites for patients with cirrhosis was also significantly greater in the presence of PVT. Evaluating a pooled estimate of risk for gastroesophageal variceal hemorrhage or ascites resulting from PVT could not be performed due to the lack of data reporting by the included studies. While this has been shown in other studies, these were not included due to a lack of a comparison group.
PVT appears to increase mortality and hepatic decompensation (composite as well as variceal hemorrhage and ascites), however, the relatively small number of included studies limits generalizable conclusions. Our study has several other limitations. Multiple studies with a large number of patients were excluded due to a lack of a comparison group, and our literature search revealed a general lack of randomized controlled trials within the context of PVT. The large multicenter prospective study by Nery et al[11] was specifically excluded due to a lack of absolute numbers and component hepatic decompensation assessment. The inclusion of only three studies also limits the systematic assessment for publication bias and may also have resulted in the large degree of heterogeneity seen in calculation of the pooled measure of effect for PVT and the development of ascites.
Regardless, this current review represents the best available summary of the evidence to date and highlights a significant need for future research around the implications of PVT, especially prospective studies with a direct comparator group. Safety and efficacy data on both prevention and treatment of PVT is in general lacking. Villa et al[21] recently published their unblinded randomized, single center experience having found that daily prophylactic dosing of low molecular weight heparin (the equivalent of 40 mg/d) for twelve months prevented the development of PVT in patients with compensated cirrhosis. While the study was terminated at 48 wk, the effect persisted through follow-up at 5 years when compared to standard of care[21]. Additionally, the authors demonstrated less hepatic decompensation in the low molecular weight heparin arm (P < 0.0001) and a more importantly, a significant survival benefit[21]. Building on this work, Cui et al[22] published their single center randomized trial of 65 patients investigating therapeutic doses of low-molecular weight heparin (1 mg/kg every twelve hours or 1.5 mg/kg daily), where 78.5% (n = 51) responded to treatment with either complete or partial recanalization at 6 mo after starting therapy. These responders had regression of their liver disease when compared directly to the 14 non-responders. Similar to Villa et al[21], Cui et al[22] found no episodes of variceal hemorrhage, however, they did find much higher rates of non-variceal bleeding (6.4%-23.5%). While this study has several limitations including its generalizability as it only enrolled hepatitis B patients in China, it is nonetheless promising. With the development of new oral anticoagulants, the promise of treatment and possibly prevention is becoming a reality[23]. More rigorous study is needed in the field of coagulation disorders in a randomized, placebo controlled interventional or preventative trial with a direct comparator group using either heparin based or new direct acting oral anticoagulant therapy.
In conclusion, PVT appears to increase mortality and hepatic decompensation from ascites, however, the relatively small number of included studies limits generalizable conclusions and contributes significant heterogeneity in the pooled measures of effect. More prospective, randomized placebo controlled trials with a direct comparator group are needed.
Non-neoplastic portal vein thrombosis is a common complication of cirrhosis. Treatment options carry risk and are not without complications. To make appropriate clinical decisions, clinicians need to be cognizant of the available evidence.
The field of coagulation disorders in chronic liver disease is ever expanding. Much of the research focuses on portal vein thrombosis and clinically relevant outcomes with the goal of preventing hepatic decompensation through either prevention or treatment of portal vein thrombosis.
In the present study, the authors investigated the impact of portal vein thrombosis on mortality and hepatic decompensation in patients with cirrhosis. This is the first report of a meta-analysis in patients with cirrhosis specifically excluding those who go on to receive liver transplantation.
The present report furthers understanding regarding the clinical importance of portal vein thrombosis in patients with underlying cirrhosis.
This systematic review and meta-analysis adds useful information for both clinical practice and further academic research with the goal of impacting patient centered outcomes.
P- Reviewer: Liang Y, Lim EC, Wang SK S- Editor: Wang JL L- Editor: A E- Editor: Liu SQ
| 1. | Kinjo N, Kawanaka H, Akahoshi T, Matsumoto Y, Kamori M, Nagao Y, Hashimoto N, Uehara H, Tomikawa M, Shirabe K. Portal vein thrombosis in liver cirrhosis. World J Hepatol. 2014;6:64-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 614] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 3. | Ogren M, Bergqvist D, Björck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol. 2006;12:2115-2119. [PubMed] [Cited in This Article: ] |
| 4. | Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238-1247. [PubMed] [Cited in This Article: ] |
| 5. | Okuda K, Ohnishi K, Kimura K, Matsutani S, Sumida M, Goto N, Musha H, Takashi M, Suzuki N, Shinagawa T. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology. 1985;89:279-286. [PubMed] [Cited in This Article: ] |
| 6. | Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, Riccardi L, Lancellotti S, Santoliquido A, Flore R. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 7. | Englesbe MJ, Kubus J, Muhammad W, Sonnenday CJ, Welling T, Punch JD, Lynch RJ, Marrero JA, Pelletier SJ. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl. 2010;16:83-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 170] [Article Influence: 12.1] [Reference Citation Analysis (2)] |
| 8. | Rodríguez-Castro KI, Porte RJ, Nadal E, Germani G, Burra P, Senzolo M. Management of nonneoplastic portal vein thrombosis in the setting of liver transplantation: a systematic review. Transplantation. 2012;94:1145-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Hibi T, Nishida S, Levi DM, Selvaggi G, Tekin A, Fan J, Ruiz P, Tzakis AG. When and why portal vein thrombosis matters in liver transplantation: a critical audit of 174 cases. Ann Surg. 2014;259:760-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Ponziani FR, Zocco MA, Senzolo M, Pompili M, Gasbarrini A, Avolio AW. Portal vein thrombosis and liver transplantation: implications for waiting list period, surgical approach, early and late follow-up. Transplant Rev (Orlando). 2014;28:92-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Nery F, Chevret S, Condat B, de Raucourt E, Boudaoud L, Rautou PE, Plessier A, Roulot D, Chaffaut C, Bourcier V. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61:660-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 12. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] [Cited in This Article: ] |
| 13. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [PubMed] [Cited in This Article: ] |
| 14. | Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14:29-37. [PubMed] [Cited in This Article: ] |
| 15. | Murad MH, Montori VM, Ioannidis JP, Jaeschke R, Devereaux PJ, Prasad K, Neumann I, Carrasco-Labra A, Agoritsas T, Hatala R. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA. 2014;312:171-179. [PubMed] [Cited in This Article: ] |
| 16. | John BV, Konjeti R, Aggarwal A, Lopez R, Atreja A, Miller C, Zein NN, Carey WD. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol. 2013;12:952-958. [PubMed] [Cited in This Article: ] |
| 17. | Maruyama H, Okugawa H, Takahashi M, Yokosuka O. De novo portal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes. Am J Gastroenterol. 2013;108:568-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [PubMed] [Cited in This Article: ] |
| 19. | Gayowski TJ, Marino IR, Doyle HR, Echeverri L, Mieles L, Todo S, Wagener M, Singh N, Yu VL, Fung JJ. A high incidence of native portal vein thrombosis in veterans undergoing liver transplantation. J Surg Res. 1996;60:333-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl. 2015;21:1016-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253-1260.e1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 490] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 22. | Cui SB, Shu RH, Yan SP, Wu H, Chen Y, Wang L, Zhu Q. Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroenterol Hepatol. 2015;27:914-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Intagliata NM, Maitland H, Northup PG, Caldwell SH. Treating thrombosis in cirrhosis patients with new oral agents: ready or not? Hepatology. 2015;61:738-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |