Published online Nov 8, 2015. doi: 10.4254/wjh.v7.i25.2563
Peer-review started: May 30, 2015
First decision: June 18, 2015
Revised: July 22, 2015
Accepted: October 16, 2015
Article in press: October 19, 2015
Published online: November 8, 2015
Cholangiocarcinoma (CC) is primarily a malignant tumor of older adults most prevalent in Southeast Asia, where liver fluke infestation is high. However the etiology in western countries is unknown. Although the incidence of extrahepatic cholangiocarcinoma has remained constant, incidence of intrahepatic CC (ICC) which differs in morphology, pathogenesis, risk factors, treatment and prognosis is increasing. While this increase is associated with hepatitis C virus infection, chronic nonalcoholic liver disease, obesity, and smoking, the pathogenesis of ICC and molecular alterations underlying the carcinogenesis are not completely elucidated. Benign biliary lesions such as biliary intraepithelial neoplasia, intraductal papillary neoplasm of the bile duct, von Meyenburg complex or bile duct hamartoma, and bile duct adenoma have been associated with ICC. For each of these entities, evidence suggests or supports a role as premalignant lesions. This article summarized the important biological significance of the precursor lesions of ICC and the molecular mechanisms that may be involved in intrahepatic cholangiocarcinogenesis.
Core tip: This manuscript highlights the important development in the research field of intrahepatic cholangiocarcinogenesis, and summarizes some key points related to progression from the precursor lesions to the intrahepatic cholangiocarcinoma, including their molecular genetics. Each individual precursor or potential precursor is linked to the cancer by the clinical, histological and molecular association.
- Citation: Ettel M, Eze O, Xu R. Clinical and biological significance of precursor lesions of intrahepatic cholangiocarcinoma. World J Hepatol 2015; 7(25): 2563-2570
- URL: https://www.wjgnet.com/1948-5182/full/v7/i25/2563.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i25.2563
Intrahepatic cholangiocarcinoma (ICC) is responsible for 10%-20% of primary liver cancers worldwide[1]. ICCs are classified as perihilar or peripheral type. The perihilar type, involving the large bile ducts, is composed of a large tubular component or papillary proliferation of tall columnar epithelium with mucin production. The peripheral type, involving the smaller ducts and segmental branches, is composed of a proliferation of relatively small, tubular, closely packed cord-like structures or ductular pattern lined by small cuboidal epithelium[2]. Grossly, both perihilar and peripheral ICC are firm and white to tan[1]. Another classification scheme divides ICCs into conventional ductal carcinoma, bile ductular type, intraductal neoplasm and rare variants[3], highlighting similarities between intraductal neoplasms and pancreatic intraductal papillary-mucinous neoplasms, and differences between bile ductular type and the conventional type on the origin of tumor cells. These classification schemes reflect the postulated cells of tumor origin with similarities between the perihilar type, conventional ductal carcinoma, bile duct type and mucin-producing type, and between the peripheral, bile ductular, cholangiolar and cholangiolocellular types[2].
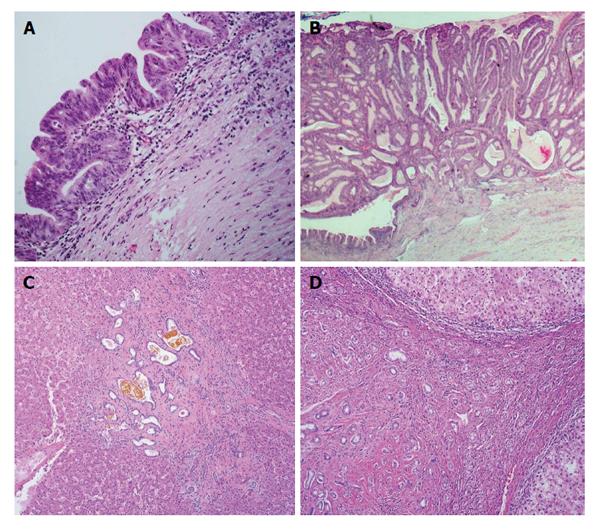
ICCs may evolve from two types of premalignant biliary lesions: Biliary intraepithelial neoplasia (BilIN) and intraductal papillary neoplasm of the bile duct (IPNB)[3] as in the case of perihilar large duct type ICCs[4]. ICC may also evolve without definite premalignant lesions. No intraepithelial or intraductal preneoplastic, dysplastic or neoplastic lesions have been demonstrated in the small bile ducts or ductules[5]. Von Meyenburg complex (VMC) is suggested to be a possible premalignant lesion of ICCs due to the occasional association of VMC with ICCs and reports of VMC-like cystic ICCs[6-8]. Progression of bile duct adenoma (BDA) to ICC has also been reported[9]. The major clinical and histological characteristic features of above lesions are summarized in Figure 1 and Table 1. In addition to these precursor lesions, incidence is also increased in patients with risk factors such as chronic viral hepatitis, infection with parasites such as clonorchis and opistorchis, and hepatolithiasis[1].
| ICC | BilIN | IPNB | VMC/BDH | BDA | |
| Incidence/prevalence | 10%-20% of primary liver cancers[1] | No published data | 9%-38% of bile duct carcinomas[28] | 5.6% of adults, 0.9% of children (autopsy series)[40] | 0.00008%-0.006% of patients (autopsy series)[52,53] |
| Risk factors | Chronic viral hepatitis, clonorchis, opistorchis, hepatolithiasis[1] | Hepatolithiasis, primary sclerosing cholangitis, choledochal cyst, autoimmune pancreatitis, chronic viral hepatitis, alcoholic cirrhosis[15,17-21] | Hepatolithiasis, clonorchis[31,32] | Congenital hepatic fibrosis, polycystic liver disease[40] | No known risk factors |
| Gross appearance | Firm, white to tan[1] | Not grossly identifiable[10] | Dilated bile ducts filled with soft, papillary white to red to tan lesions without invasion[10] | Well-circumscribed unencapsulated nodules, < 5 mm[1] | Subcapsular, well-circumscribed unencapsulated gray to white, yellow or tan firm nodules, ≤ 2 cm[47,48] |
| Histologic appearance | Perihilar type: Involves large bile ducts, composed of large tubules or papillae lined by columnar epithelium. Peripheral type: Involves smaller ducts and segmental branches, composed of small, tubular cords or ductular pattern lined by cuboidal epithelium[2] | Epithelium with nuclear pseudostratification and atypia (increasing from BilIN-1 to BilIN-2 to BilIN-3), often with micropapillary projections into the bile duct lumen[10] | Noninvasive papillary or villous biliary neoplasm covering delicate fibrovascular stalks (subtypes pancreatobiliary, intestinal, gastric, oncocytic)[10] | Irregular dilated to branching low cuboidal epithelium-lined ductules within fibrous stroma, often adjacent to portal areas[1] | Small uniform cuboidal epithelium-lined ductules within fibrous stroma[47,48] |
| Molecular alterations | Activating mutations in KRAS (22%) occurs early in cholangiocarcinogenesis[63] | Activating mutation of KRAS present in approximately 33% of BilIN lesions including in 25% of cases of BilIN-1[22] | Increased expression of Cyclin D1 and p21[26,35] | Loss of heterozygosity at key loci (5q21, 9p21, 10q23, 17p13) harboring APC, p53, p16, and PTEN[44] | BRAF V600E mutation (53%)[46] |
| Loss-of-function mutations in TP53 (15%), BRAF and EGFR mutation (7% and 2%)[55,61,65,67] | Increased expression of p21, p53, cyclin D1 and EZH2[3,17,22-25] | Aberrant expression of p16[30] | |||
| Rare NRAS and PI3K mutations have been[55,61-72] | Decreased expression of Dcp4 and p16INK4A. Loss of SMAD4/DPC4 associated with higher grade[3,30,35] | Inactivation of p53 associated with increasing grade of dysplasia and invasion[30] | |||
| IDH1 and IDH2 mutations co-occurring with increased TP53 expression and associated with DNA hypermethylation[62,66] | Decreased membranous expression of β-catenin with increasing grade of BilIN[26] | C-myc mutations in over 50% of cases[26] | |||
| Chromosomal aberrations including gains at 7p and 8q and losses at 1p, 4q and 9p[68,69,73-76] | Decreased expression of E-cadherin in some cases of BilIN[26] | Loss of SMAD4/DPC4 associated with higher grade[30,35] | |||
| Aberrant methylation of p16INK4a/CDKN2 (47%), RASSFIA (56%) and APC (29%)[70,71,76] | S100P: Increased immunohistochemical expression in BilIN-2 and BilIN-3[27] |
BilIN is an epithelial lesion and a precursor to both ICCs (the intrahepatic bile ducts and peribiliary gland) and to extrahepatic cholangiocarcinoma (the extrahepatic bile ducts and gallbladder)[10,11]. BilIN is characterized by epithelial cells with nuclear pseudostratification and atypia, often with micropapillary projections into the bile duct lumen[10]. It is not grossly identifiable[10]. BilIN has often been described as the biliary counterpart to pancreatic intraepithelial neoplasia (PanIN), partially due to the similar embryonal development and morphology of the pancreatic and biliary duct systems[12]. Grading of BilIN follows a similar 3-tiered pattern to that of PanIN, which has proven similar in several morphologic and immunohistochemical respects[13]. BilIN is subdivided into BilIN-1, BilIN-2 and BilIN-3 according to consensus criteria proposed in 2005 and accepted by an international group in 2007[14,15]. BilIN-1 shows relatively uniform, mildly irregular nuclei confined to the lower two thirds of the epithelium. In BilIN-2, nuclei are full-thickness and can be found apically, and loss of cellular and nuclear polarity is present but not diffuse. Nuclear irregularity is increased compared to BilIN-1, but mitoses remain rare. BilIN-3 is notable for marked cellular and nuclear atypia and loss of polarity, with cytological resemblance to invasive cholangiocarcinoma albeit without basement membrane invasion. Cribriforming and budding of cell clusters as well as mitoses are often seen[11,15]. Macroscopic and radiologic identification of these lesions is not possible[11].
BilIN has been associated with several conditions which can predispose to cholangiocarcinoma. There is no solid datum about the prevalence of BilIN. While estimates of overall prevalence of BilIN are difficult to determine, one study for margin status in biliary carcinomas showed that 4 of 19 ICCs had BilIN at the margin[16].
BilIN is frequently diagnosed in cases of hepatolithiasis[17], and primary sclerosing cholangitis and choledochal cyst have also been associated with BilIN[15,18]. BilIN has also been shown to arise in autoimmune pancreatitis, chronic hepatitis B and C, and alcoholic cirrhosis[19-21].
BilIN shares several molecular alterations with cholangiocarcinoma, and study of BilIN alongside cholangiocarcinoma has helped to elucidate several of the key molecular mechanisms in cholangiocarcinogenesis. Many of these molecular changes accumulate in conjunction with increasing grade of BilIN[11]. KRAS has been shown to occur early in cholangiocarcinogenesis and is present in approximately 33% of BilIN lesions including in 25% of cases of BilIN-1[22]. Also, p21, p53, cyclin D1 and EZH2 expression has been shown to increase and expression of Dcp4 and p16INK4A decreases in tandem with increasing grade of BilIN[3]. Importantly, p53 overexpression has been shown in BilIN-3 but reports show no increased expression in BilIN-1/2[17,22]. Overexpression of EZH2 has been related to hypermethylation of the p16 promoter, which could explain the correlating increase in EZH2 expression and decrease of p16 expression with increasing grade of BilIN[23]. Overexpression in EZH2 and decreased p16 expression are associated with deregulation of cellular senescence, and deregulation of autophagy has been shown through in vitro studies to precede cellular senescence[24,25]. Related to these findings, it is notable that a recent study of expression of autophagy-related proteins showed that light chain 3β (LC3), beclin-1 and p62 showed increased expression in BilIN-1, BilIN-2 and BilIN-3, suggesting that deregulated autophagy plays a role in occurrence and development of BilIN[17]. Decreased membranous expression of β-catenin similarly correlates with increasing grade of BilIN, while E-cadherin is decreased in some cases of BilIN but in a smaller proportion of cases compared to invasive cholangiocarcinoma[26]. S100P also showed increased immunohistochemical expression in BilIN-2 and BilIN-3 as compared to reactive benign epithelium and BilIN-1[27]. The S100P immunohistochemical marker is of particular interest as it has been suggested for use in clinical diagnosis and grading of BilIN[11].
IPNB, defined as a precursor to cholangiocarcinoma according to the World Health Organization 2010 classification, has been described as the biliary counterpart to the intraductal papillary mucinous neoplasm (IPMN) of the pancreas[10,28]. Similarly to IPMN, the neoplasm is characterized by thin fibrovascular cores lined with noninvasive papillary or villous epithelium and filling a dilated bile duct lumen[10]. Grossly, IPNB shows dilated, fusiform to cystic bile ducts with soft papillary lesions ranging from white to tan to red. Histologically, the aforementioned fibrovascular cores are lined by any of four cell types: Pancreatobiliary, intestinal, gastric and oncocytic types, parallel to those defined in IPMN[10]. However, there are important differences between IPNB and IPMN. Only approximately 1/3 of IPNB show macroscopic mucin production, while only rare IPMNs are not associated with mucin hypersecretion[28,29]. Also, pancreatobiliary type epithelium is most common and gastric type epithelium is rare in IPNB, whereas IPMN least commonly shows pancreatobiliary differentiation[10] and gastric is among the more common patterns[30]. Just as grading of BilIN parallels that of PanIN, grading in IPNB follows that of IPMN. Low, intermediate and high grade are assigned based on cytologic and architectural characteristics, where low- and intermediate-grade tumors comprise one diagnostic entity, high-grade tumors another, and IPNB with associated invasive carcinoma a final separate entity[10]. Studies of histomorphologic, molecular and other features of these tumors have shown disparate results to some extent due to the different features of IPNB with different etiology.
IPNB comprises 9%-38% of bile duct carcinomas and is most common in patients from far eastern countries in their 6th and 7th decades[28]. In these patients who often have risk factors such as hepatolithiasis and clonorchiasis, intestinal subtype is most common (47% and 38% in two large series)[31,32]. However, Western cohorts more commonly show pancreatobiliary type epithelium (36%, 69% and 45% of patients in three recent series from the United States and western Europe)[30,33,34]. Series in all populations have shown uncommon oncocytic and gastric subtypes.
Molecular changes in IPNB remain poorly characterized, although several recent studies have begun to explore various molecular mechanisms in these precursor neoplasms. Comparisons between IPNB and BilIN have shown conflicting results. Cyclin D1 and p21 expression increase occurs in IPNB just as it does in in BilIN; a study by Itatsu et al[26] showed higher expression in IPNB than in BilIN while Nakanishi et al[35] did not find this differential expression. Another cell cycle protein, p16, has also shown aberrant expression in IPNB and this aberrant expression was shown to be more frequent in cholangiocarcinoma than in IPNB[30]. Inactivation of p53 has been shown to increase with increasing grade of dysplasia and with invasion in one study[30], while other studies have shown different results[35,36]. C-myc mutations have been shown to be common as well with expression in over half of cases in one study[26]. Similar to pancreatic neoplasms, loss of SMAD4/DPC4 has been shown in IPNB and BilIN with increased loss associated with higher grade[30,35]. GNAS mutation has been seen mostly in intestinal subtype and mucin hypersecretion-associated cases, and has been recognized more frequently in studies involving Asian cohorts where intestinal subtype is more common[30,37]. KRAS mutations on the other hand appear more common in early and low-grade lesions[28,30].
VMCs, also known variously as bile duct or biliary hamartomas or as biliary microhamartomas, are small (generally < 5 mm), well-circumscribed unencapsulated nodules which consist of irregularly shaped, often dilated bile ducts lined by a low cuboidal epithelium, embedded within a dense collagenous stroma. VMCs are thought to result from persistence of the embryonic ductal plate and are generally adjacent to portal areas[1]. Multiple case reports showed exist of VMCs with histologic evidence of transformation to cholangiocarcinoma, showing benign, hyperplastic and dysplastic epithelium with adjacent progression to invasive carcinoma[7,8,38,39].
These lesions are fairly common with prevalence of 5.6% in adults and 0.9% of children in one autopsy series, and are especially prevalent in patients with adult polycystic kidney disease or congenital hepatic fibrosis[40]. VMCs are generally considered benign, but several case reports suggest that these lesions are capable of transformation to cholangiocarcinoma, showing benign, hyperplastic and dysplastic epithelium with adjacent progression to invasive carcinoma, particularly in patients with multiple VMCs[8,38,39,41-43]. This phenomenon has been corroborated with molecular evidence of mutations shared between cholangiocarcinoma and VMCs[44]. Further suggesting this link is a radiologic study of 6 patients with multiple VMCs in which one of the six (17%) patients with initial imaging evidence of VMCs developed cholangiocarcinoma within 2 years[45].
Specific molecular genetic changes for transformation of VMCs to cholangiocarcinoma have not been definitively established[38]. In contrast to biliary duct adenomas, BRAF V600E mutations seen in a subset of ICC are not present in VMCs[46]. However, recent studies have identified multiple findings suggestive of the sequential genetic changes required for transformation of benign VMCs to invasive cholangiocarcinoma[38,44]. In one study of two patients with multiple VMCs and cholangiocarcinoma, loss of heterozygosity (LOH) was examined at 20 key genetic loci in the cholangiocarcinoma tumor, in VMCs distant from the tumor, and in intermediate lesions where VMCs showed transformation to cholangiocarcinoma. LOH was seen in VMCs at some of the same key loci as seen in cholangiocarcinoma, affecting oncogenes p16, p53, APC and PTEN which have been shown to play a role in the development of cholangiocarcinoma[44]. In contrast, immunohistochemical expression of p16INK4A was shown to be lost in cholangiocarcinoma in one report while adjacent uninvolved VMC ducts retained expression of p16INK4A[38].
BDA are small (< 2 cm) benign gray to white, yellow or tan lesions usually located directly beneath the liver capsule. They are firm, well-circumscribed and not encapsulated. Histologically, they are comprised of uniform tubular or curvilinear ductules within a fibrous stroma[47,48]. The ductules are lined by cuboidal cells with bland, round to oval nuclei and without mitotic activity, and sometimes show mucinous metaplasia, 1-antitrypsin droplets and neuroendocrine differentiation. Previously synonymous with bile duct hamartoma (BDH)[49] - BDA and BDH have overlapping features - histological findings and immunohistochemical properties aid in distinguishing between the two. It has been reported that BDAs originate from peri-biliary glands and not bile ductules or ducts[50]. This finding is supported by the monoclonal antibody identification of antigens D10 and IF6 and the presence of mucin cells; a shared profile amongst peribiliary glands and BDAs but not VMCs. Malignant transformation of BDA has not been unequivocally demonstrated. ICC in a background of BDA mixed with minor component of BDH is reported in the literature[51] (also present in authors’ unpublished case report). There has also been a report of progression of BDA to ICC[9]. BDA is a rare lesion and has been found in 0.0008%-0.006% of patients in two large autopsy series[52,53].
Molecular characterization of BDAs is incomplete. The BRAF V600E mutation was identified by PCR and immunohistochemistry in 53% (8/15) cases of BDA in one series[46]. Interestingly, both wild-type BRAF and BRAF V600E lesions coexisted in patients with multiple BDAs. Identification of oncogenic mutations in BDA supports a benign neoplasm rather than reactive process and suggests that BDA may be an early lesion in the pathogenesis of ICC, which has been shown to harbor BRAF V600E mutations[54,55]. Furthermore, the finding of coexistence of benign lesions, dysplastic lesions and carcinoma (authors’ unpublished data) and progression of BDA to ICC[9] strongly suggests an adenoma-dysplasia-carcinoma pathway, as seen in colorectal carcinogenesis. BRAF V600E mutation was not identified in von Meyenberg complexes, supporting distinct etiologies with different molecular and biological processes[46].
Few cases of biliary adenofibroma[56-59], a benign tumor with complex tubulocystic nonmucin secreting biliary epithelial and an abundant fibroblastic stromal components, have been reported to date. The immunohistochemical profile (cytokeratins 7, 8, 18, 19 and D10 positive, and 1F6 negative) suggests a large bile duct or interlobular duct origin[59,60]. Malignant transformation of the epithelial component has been reported[56].
Molecular alterations underlying ICC and the premalignant biliary lesions are not completely elucidated. Activating mutations in KRAS (22%), loss-of-function mutations in TP53 (15%), BRAF and EGFR mutation (7% and 2%), and rare NRAS and PI3K mutations have been reported[55,61-72]. IDH1 and IDH2 mutations co-occurring with increased TP53 expression and associated with DNA hypermethylation has also been reported, although the functional relevance is unknown. Chromosomal aberrations including gains at 7p and 8q and losses at 1p, 4q and 9p were identified by comparative genomic hybridization studies[69,73-76]. Aberrant methylation of p16INK4a/CDKN2 (47%), RASSFIA (56%) and APC (29%) genes have been demonstrated in ICC although the exact role in the pathogenesis is also not delineated. Investigation of genetic and epigenetic alterations in benign intrahepatic biliary lesions will further highlight mechanisms of carcinogenesis.
P- Reviewer: Shi ZJ, Yagi H S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Goodman Z, Terracciano L, Wee A. Tumours and tumour-like lesions of the liver. MacSween’s Pathology of the Liver. 6th ed. Edinburgh: Elsevier 2012; . [Cited in This Article: ] |
| 2. | Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Nakanuma Y, Sasaki M, Sato Y, Ren X, Ikeda H, Harada K. Multistep carcinogenesis of perihilar cholangiocarcinoma arising in the intrahepatic large bile ducts. World J Hepatol. 2009;1:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Nakanuma Y, Tsutsui A, Ren XS, Harada K, Sato Y, Sasaki M. What are the precursor and early lesions of peripheral intrahepatic cholangiocarcinoma? Int J Hepatol. 2014;2014:805973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Aishima S, Nishihara Y, Tsujita E, Taguchi K, Soejima Y, Taketomi A, Ikeda Y, Maehara Y, Tsuneyoshi M. Biliary neoplasia with extensive intraductal spread associated with liver cirrhosis: a hitherto unreported variant of biliary intraepithelial neoplasia. Hum Pathol. 2008;39:939-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Blanc JF, Bernard PH, Carles J, Le Bail B, Balabaud C, Bioulac-Sage P. Cholangiocarcinoma arising in Von Meyenburg complex associated with hepatocellular carcinoma in genetic haemochromatosis. Eur J Gastroenterol Hepatol. 2000;12:233-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Jain D, Sarode VR, Abdul-Karim FW, Homer R, Robert ME. Evidence for the neoplastic transformation of Von-Meyenburg complexes. Am J Surg Pathol. 2000;24:1131-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Song JS, Lee YJ, Kim KW, Huh J, Jang SJ, Yu E. Cholangiocarcinoma arising in von Meyenburg complexes: report of four cases. Pathol Int. 2008;58:503-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Pinho AC, Melo RB, Oliveira M, Almeida M, Lopes J, Graça L, Costa-Maia J. Adenoma-carcinoma sequence in intrahepatic cholangiocarcinoma. Int J Surg Case Rep. 2012;3:131-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Bosman F, Carneiro F, Hruban R, Theise N. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press 2010; . [Cited in This Article: ] |
| 11. | Sato Y, Sasaki M, Harada K, Aishima S, Fukusato T, Ojima H, Kanai Y, Kage M, Nakanuma Y, Tsubouchi H. Pathological diagnosis of flat epithelial lesions of the biliary tract with emphasis on biliary intraepithelial neoplasia. J Gastroenterol. 2014;49:64-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Nakanuma Y, Harada K, Sasaki M, Sato Y. Proposal of a new disease concept “biliary diseases with pancreatic counterparts”. Anatomical and pathological bases. Histol Histopathol. 2014;29:1-10. [PubMed] [Cited in This Article: ] |
| 13. | Sato Y, Harada K, Sasaki M, Nakanuma Y. Histological Characterization of Biliary Intraepithelial Neoplasia with respect to Pancreatic Intraepithelial Neoplasia. Int J Hepatol. 2014;2014:678260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Zen Y, Aishima S, Ajioka Y, Haratake J, Kage M, Kondo F, Nimura Y, Sakamoto M, Sasaki M, Shimamatsu K. Proposal of histological criteria for intraepithelial atypical/proliferative biliary epithelial lesions of the bile duct in hepatolithiasis with respect to cholangiocarcinoma: preliminary report based on interobserver agreement. Pathol Int. 2005;55:180-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Zen Y, Adsay NV, Bardadin K, Colombari R, Ferrell L, Haga H, Hong SM, Hytiroglou P, Klöppel G, Lauwers GY. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20:701-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Matthaei H, Lingohr P, Strässer A, Dietrich D, Rostamzadeh B, Glees S, Roering M, Möhring P, Scheerbaum M, Stoffels B. Biliary intraepithelial neoplasia (BilIN) is frequently found in surgical margins of biliary tract cancer resection specimens but has no clinical implications. Virchows Arch. 2015;466:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Sasaki M, Nitta T, Sato Y, Nakanuma Y. Autophagy may occur at an early stage of cholangiocarcinogenesis via biliary intraepithelial neoplasia. Hum Pathol. 2015;46:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Katabi N, Pillarisetty VG, DeMatteo R, Klimstra DS. Choledochal cysts: a clinicopathologic study of 36 cases with emphasis on the morphologic and the immunohistochemical features of premalignant and malignant alterations. Hum Pathol. 2014;45:2107-2114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Ohtani H, Ishida H, Ito Y, Yamaguchi T, Koizumi M. Autoimmune pancreatitis and biliary intraepithelial neoplasia of the common bile duct: a case with diagnostically challenging but pathogenetically significant association. Pathol Int. 2011;61:481-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Aishima S, Iguchi T, Fujita N, Taketomi A, Maehara Y, Tsuneyoshi M, Oda Y. Histological and immunohistological findings in biliary intraepithelial neoplasia arising from a background of chronic biliary disease compared with liver cirrhosis of non-biliary aetiology. Histopathology. 2011;59:867-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Wu TT, Levy M, Correa AM, Rosen CB, Abraham SC. Biliary intraepithelial neoplasia in patients without chronic biliary disease: analysis of liver explants with alcoholic cirrhosis, hepatitis C infection, and noncirrhotic liver diseases. Cancer. 2009;115:4564-4575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Hsu M, Sasaki M, Igarashi S, Sato Y, Nakanuma Y. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer. 2013;119:1669-1674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Sasaki M, Yamaguchi J, Itatsu K, Ikeda H, Nakanuma Y. Over-expression of polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J Pathol. 2008;215:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavaré S, Arakawa S, Shimizu S, Watt FM. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 773] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 25. | Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Autophagy mediates the process of cellular senescence characterizing bile duct damages in primary biliary cirrhosis. Lab Invest. 2010;90:835-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Itatsu K, Zen Y, Ohira S, Ishikawa A, Sato Y, Harada K, Ikeda H, Sasaki M, Nimura Y, Nakanuma Y. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007;27:1174-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Aishima S, Fujita N, Mano Y, Kubo Y, Tanaka Y, Taketomi A, Shirabe K, Maehara Y, Oda Y. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol. 2011;35:590-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, Sakai Y, Yokosuka O, Miyazaki M. Intraductal papillary neoplasms of the bile duct. Int J Hepatol. 2014;2014:459091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Suda K. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011;35:512-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Schlitter AM, Born D, Bettstetter M, Specht K, Kim-Fuchs C, Riener MO, Jeliazkova P, Sipos B, Siveke JT, Terris B. Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Mod Pathol. 2014;27:73-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Kim KM, Lee JK, Shin JU, Lee KH, Lee KT, Sung JY, Jang KT, Heo JS, Choi SH, Choi DW. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012;107:118-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 32. | Ji Y, Fan J, Zhou J, Wang BS, Liu HB, Wu ZW, Tan YS. Intraductal papillary neoplasms of bile duct. A distinct entity like its counterpart in pancreas. Histol Histopathol. 2008;23:41-50. [PubMed] [Cited in This Article: ] |
| 33. | Kloek JJ, van der Gaag NA, Erdogan D, Rauws EA, Busch OR, Gouma DJ, ten Kate FJ, van Gulik TM. A comparative study of intraductal papillary neoplasia of the biliary tract and pancreas. Hum Pathol. 2011;42:824-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 173] [Article Influence: 14.4] [Reference Citation Analysis (2)] |
| 35. | Nakanishi Y, Zen Y, Kondo S, Itoh T, Itatsu K, Nakanuma Y. Expression of cell cycle-related molecules in biliary premalignant lesions: biliary intraepithelial neoplasia and biliary intraductal papillary neoplasm. Hum Pathol. 2008;39:1153-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Abraham SC, Lee JH, Hruban RH, Argani P, Furth EE, Wu TT. Molecular and immunohistochemical analysis of intraductal papillary neoplasms of the biliary tract. Hum Pathol. 2003;34:902-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Sasaki M, Matsubara T, Yoneda N, Nomoto K, Tsuneyama K, Sato Y, Nakanuma Y. Overexpression of enhancer of zeste homolog 2 and MUC1 may be related to malignant behaviour in intraductal papillary neoplasm of the bile duct. Histopathology. 2013;62:446-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Parekh V, Peker D. Malignant Transformation in Von-Meyenburg Complexes: Histologic and Immunohistochemical Clues With Illustrative Cases. Appl Immunohistochem Mol Morphol. 2015;23:607-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Xu AM, Xian ZH, Zhang SH, Chen XF. Intrahepatic cholangiocarcinoma arising in multiple bile duct hamartomas: report of two cases and review of the literature. Eur J Gastroenterol Hepatol. 2009;21:580-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Redston MS, Wanless IR. The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies [corrected]. Mod Pathol. 1996;9:233-237. [PubMed] [Cited in This Article: ] |
| 41. | Neto AG, Dainiak C, Qin L, Salem RR, Jain D. Intraductal papillary cholangiocarcinoma associated with von Meyenberg complexes: a case report. Dig Dis Sci. 2007;52:2643-2645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Orii T, Ohkohchi N, Sasaki K, Satomi S, Watanabe M, Moriya T. Cholangiocarcinoma arising from preexisting biliary hamartoma of liver--report of a case. Hepatogastroenterology. 2003;50:333-336. [PubMed] [Cited in This Article: ] |
| 43. | Röcken C, Pross M, Brucks U, Ridwelski K, Roessner A. Cholangiocarcinoma occurring in a liver with multiple bile duct hamartomas (von Meyenburg complexes). Arch Pathol Lab Med. 2000;124:1704-1706. [PubMed] [Cited in This Article: ] |
| 44. | Jain D, Ahrens W, Finkelstein S. Molecular evidence for the neoplastic potential of hepatic Von-Meyenburg complexes. Appl Immunohistochem Mol Morphol. 2010;18:166-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Luo TY, Itai Y, Eguchi N, Kurosaki Y, Onaya H, Ahmadi Y, Niitsu M, Tsunoda HS. Von Meyenburg complexes of the liver: imaging findings. J Comput Assist Tomogr. 1998;22:372-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 46. | Pujals A, Bioulac-Sage P, Castain C, Charpy C, Zafrani ES, Calderaro J. BRAF V600E mutational status in bile duct adenomas and hamartomas. Histopathology. 2015;67:562-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Ferrell L. Benign and Malignant Tumors of the Liver. Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas. 3rd ed. Philadelphia: Elsevier 2014; . [Cited in This Article: ] |
| 48. | Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988;12:708-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 127] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Börnfors M. The development of cholangiocarcinoma from multiple bile-duct adenomas. Report of a case and review of the literature. Acta Pathol Microbiol Immunol Scand A. 1984;92:285-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 50. | Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996;20:858-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Hasebe T, Sakamoto M, Mukai K, Kawano N, Konishi M, Ryu M, Fukamachi S, Hirohashi S. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch. 1995;426:209-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Edmonson HA. Tumors of the liver and intrahepatic bile ducts. Washington, DC: Armed Forces Institute of Pathology 1958; 18-24, 179-190. [Cited in This Article: ] |
| 53. | Cho C, Rullis I, Rogers LS. Bile duct adenomas as liver nodules. Arch Surg. 1978;113:272-274. [PubMed] [Cited in This Article: ] |
| 54. | Goeppert B, Frauenschuh L, Renner M, Roessler S, Stenzinger A, Klauschen F, Warth A, Vogel MN, Mehrabi A, Hafezi M. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod Pathol. 2014;27:1028-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 55. | Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, Hauss J, Wittekind C. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 280] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 56. | Akin O, Coskun M. Biliary adenofibroma with malignant transformation and pulmonary metastases: CT findings. AJR Am J Roentgenol. 2002;179:280-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Parada LA, Bardi G, Hallén M, Hägerstrand I, Tranberg KG, Mitelman F, Johansson B. Monosomy 22 in a case of biliary adenofibroma. Cancer Genet Cytogenet. 1997;93:183-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Tsui WM, Loo KT, Chow LT, Tse CC. Biliary adenofibroma. A heretofore unrecognized benign biliary tumor of the liver. Am J Surg Pathol. 1993;17:186-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Varnholt H, Vauthey JN, Dal Cin P, Marsh Rde W, Bhathal PS, Hughes NR, Lauwers GY. Biliary adenofibroma: a rare neoplasm of bile duct origin with an indolent behavior. Am J Surg Pathol. 2003;27:693-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Gurrera A, Alaggio R, Leone G, Aprile G, Magro G. Biliary adenofibroma of the liver: report of a case and review of the literature. Patholog Res Int. 2010;2010:504584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021-1031.e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 379] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 62. | Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 555] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 63. | Chen TC, Jan YY, Yeh TS. K-ras mutation is strongly associated with perineural invasion and represents an independent prognostic factor of intrahepatic cholangiocarcinoma after hepatectomy. Ann Surg Oncol. 2012;19 Suppl 3:S675-S681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Deshpande V, Nduaguba A, Zimmerman SM, Kehoe SM, Macconaill LE, Lauwers GY, Ferrone C, Bardeesy N, Zhu AX, Hezel AF. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer. 2011;11:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Furubo S, Harada K, Shimonishi T, Katayanagi K, Tsui W, Nakanuma Y. Protein expression and genetic alterations of p53 and ras in intrahepatic cholangiocarcinoma. Histopathology. 1999;35:230-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Kipp BR, Voss JS, Kerr SE, Barr Fritcher EG, Graham RP, Zhang L, Highsmith WE, Zhang J, Roberts LR, Gores GJ. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43:1552-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 67. | Leone F, Cavalloni G, Pignochino Y, Sarotto I, Ferraris R, Piacibello W, Venesio T, Capussotti L, Risio M, Aglietta M. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin Cancer Res. 2006;12:1680-1685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 68. | Momoi H, Itoh T, Nozaki Y, Arima Y, Okabe H, Satoh S, Toda Y, Sakai E, Nakagawara K, Flemming P. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, Peix J, Sole M, Tovar V, Alsinet C. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 387] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 70. | Tannapfel A, Benicke M, Katalinic A, Uhlmann D, Köckerling F, Hauss J, Wittekind C. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Tannapfel A, Sommerer F, Benicke M, Weinans L, Katalinic A, Geissler F, Uhlmann D, Hauss J, Wittekind C. Genetic and epigenetic alterations of the INK4a-ARF pathway in cholangiocarcinoma. J Pathol. 2002;197:624-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Tannapfel A, Weinans L, Geissler F, Schütz A, Katalinic A, Köckerling F, Hauss J, Wittekind C. Mutations of p53 tumor suppressor gene, apoptosis, and proliferation in intrahepatic cholangiocellular carcinoma of the liver. Dig Dis Sci. 2000;45:317-324. [PubMed] [Cited in This Article: ] |
| 73. | Homayounfar K, Gunawan B, Cameron S, Haller F, Baumhoer D, Uecker S, Sander B, Ramadori G, Lorf T, Füzesi L. Pattern of chromosomal aberrations in primary liver cancers identified by comparative genomic hybridization. Hum Pathol. 2009;40:834-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Koo SH, Ihm CH, Kwon KC, Park JW, Kim JM, Kong G. Genetic alterations in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Genet Cytogenet. 2001;130:22-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Uhm KO, Park YN, Lee JY, Yoon DS, Park SH. Chromosomal imbalances in Korean intrahepatic cholangiocarcinoma by comparative genomic hybridization. Cancer Genet Cytogenet. 2005;157:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Wong N, Li L, Tsang K, Lai PB, To KF, Johnson PJ. Frequent loss of chromosome 3p and hypermethylation of RASSF1A in cholangiocarcinoma. J Hepatol. 2002;37:633-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |