Published online Sep 18, 2015. doi: 10.4254/wjh.v7.i20.2274
Peer-review started: August 30, 2014
First decision: November 14, 2014
Revised: July 6, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 18, 2015
Processing time: 382 Days and 13.3 Hours
Hepatocellular carcinoma (HCC) is one of the major malignant diseases in many healthcare systems. The growing number of new cases diagnosed each year is nearly equal to the number of deaths from this cancer. Worldwide, HCC is a leading cause of cancer-related deaths, as it is the fifth most common cancer and the third most important cause of cancer related death in men. Among various risk factors the two are prevailing: viral hepatitis, namely chronic hepatitis C virus is a well-established risk factor contributing to the rising incidence of HCC. The epidemic of obesity and the metabolic syndrome, not only in the United States but also in Asia, tend to become the leading cause of the long-term rise in the HCC incidence. Today, the diagnosis of HCC is established within the national surveillance programs in developed countries while the diagnosis of symptomatic, advanced stage disease still remains the characteristic of underdeveloped countries. Although many different staging systems have been developed and evaluated the Barcelona-Clinic Liver Cancer staging system has emerged as the most useful to guide HCC treatment. Treatment allocation should be decided by a multidisciplinary board involving hepatologists, pathologists, radiologists, liver surgeons and oncologists guided by personalized -based medicine. This approach is important not only to balance between different oncologic treatments strategies but also due to the complexity of the disease (chronic liver disease and the cancer) and due to the large number of potentially efficient therapies. Careful patient selection and a tailored treatment modality for every patient, either potentially curative (surgical treatment and tumor ablation) or palliative (transarterial therapy, radioembolization and medical treatment, i.e., sorafenib) is mandatory to achieve the best treatment outcome.
Core tip: In response to the hepatocellular carcinoma (HCC) burden marked differences between countries are reflected in providing disparate quality of healthcare considering screening and surveillance programs; available treatment modalities and drugs; reimbursement of specific treatment options by the state-funded health insurance. Since the number of new HCC cases being diagnosed each year is nearly equal to the number of deaths from this cancer it is clear that the international scientific community and healthcare systems worldwide have no efficient answer to this problem. International consensus on the use of any given staging model is lacking. High-quality trials with better patients’ stratification are mandatory. This review article reflects the perspective of liver surgeons working in a developing country.
- Citation: Galun D, Basaric D, Zuvela M, Bulajic P, Bogdanovic A, Bidzic N, Milicevic M. Hepatocellular carcinoma: From clinical practice to evidence-based treatment protocols. World J Hepatol 2015; 7(20): 2274-2291
- URL: https://www.wjgnet.com/1948-5182/full/v7/i20/2274.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i20.2274
According to Bailar et al[1] cancer mortality rates have not been significantly reduced in industrialized countries except for testicular cancer, leukemia and lymphoma in spite of an evident progress in developing innovative approaches for cancer treatment. Hepatocellular carcinoma (HCC) is a frustrating example for general disappointment with the results of cancer treatment having in mind that the growing number of new cases being diagnosed each year is nearly equal to the number of deaths from this disease[2-4].
Hepatocellular cancer is characterized by high and increasing incidence, late diagnosis when curative intent treatments are not feasible, low resectability rate, high recurrence after a curative intent surgery, poor response to medical treatments, and finally grave prognosis. These characteristics define HCC as one of the major malignant diseases in many healthcare systems worldwide. Today, HCC is one of the leading causes of cancer-related deaths, as it is the fifth most common cancer and the third most important cause of cancer related deaths in men[2-4].
A growing incidence of HCC was found in North America increasing annually by 5.4% between 2002 and 2006 being one of only four malignancies demonstrating a growing number of new cases[3,5]. Hispanics and blacks are found to have the greatest increase in incidence in the United States[6]. The overall 5-year survival less than 12% and 3-fold increase in incidence of HCC from 1975-2007 in both sexes made HCC the fastest rising cause of cancer related death in United States[7].
More than 748000 new cases are diagnosed each year, accounting for 9.2% of all new cancer cases worldwide (7.9% in men; 3.7% in women)[8-10]. Moreover, the number of new cases of HCC increases continuously[11].
Furthermore, HCC is a major burden for healthcare systems in underdeveloped countries with 84% of the world HCC population having the highest annual fatality ratio of any human tumor (0.96)[8,9,11]. Underdeveloped regions may even have a 100-fold greater incidence of HCC compared to developed countries. This is one of the greatest differences recorded among cancers[10]. Sub-Saharan Africa and Eastern Asia are regions with the greatest incidence of HCC demonstrating incidence rates of over 20 per 100000 individuals[7]. This figure is most probably even larger when considering that many HCC cases remain under-diagnosed or under-reported[11]. In these regions the most common cause for HCC is HBV transmission at birth and the diagnosis is established about one decade earlier compared to the developed countries characterized by HCV acquired later in life as a dominant cause for HCC[7].
Mediterranean countries have intermediate incidence rates of 10-20 per 100000 individuals, while North and South America have a relatively low incidence despite of the reported increase in the number of HCC cases (< 5 per 100000 individuals)[7].
In developed countries, HCC dominantly occurs in patients over 60 years old while in underdeveloped regions the HCC diagnosis is already established in many patients in their 30 s[9-11]. In all regions, there is a predominance of the male over the female gender (3/4:1) in the Asia-Pacific region, sub-Saharan Africa and medium-risk countries, compared to 2:1 in regions with a low incidence of HCC[8-11].
The majority of HCC cases occur in cirrhotic livers[10,12,13]; therefore the competing mortality risks from the tumor and the cirrhosis should be considered when deciding for a specific treatment modality.
In the majority of countries worldwide the diagnosis is established late when only limited treatment options are available resulting in poor treatment outcome. Only in Japan the strict adherence to the national surveillance program led to improved treatment results. This is mainly because approximately 20% of HCC cases are diagnosed in an early stage when curative treatment modalities can be applied[14,15].
In response to the HCC burden marked differences between countries worldwide are reflected in providing disparate quality of healthcare considering screening and surveillance programs; available treatment modalities and drugs; reimbursement of specific treatment options by the state-funded health insurance.
Viral hepatitis, namely chronic hepatitis C virus (HCV) is a well established risk factor contributing to the rising incidence of HCC[16]. The epidemic of obesity and the metabolic syndrome, not only in the United Stated[17] but also in Asia[18], tend to become the leading cause of the long-term rise in HCC incidence.
HCV is an important global risk factor for HCC, especially in developed countries, compiling more than 170 million of people being chronically infected worldwide[19,20]. The dominant prevalence is among injecting drug users (60%-90%); hemophiliacs (50%-70%); hemodialysis patients (15%-60%); and patients who received blood transfusions before 1991 (5%-10%)[21]. About 25% of patients having chronic HCV infection will develop cirrhosis and significant proportion will progress to HCC with a time interval of about 20 years or longer[19-21].
HCV-related carcinogenesis is mediated by inducing hepatic inflammation and later fibrosis; and finally by promoting malignant transformation of infected cells[22].
Approximately 55% of all worldwide HCC cases are associated with chronic hepatitis B virus (HBV) infection[8]. Among 400 million people chronically infected with HBV, about 25% will develop HCC[2,8]. Chronic HBV infection distribution is nearly parallel to HCC high-risk regions and it is implicated in the development of 85% of HCC cases among ethnic Chinese and the Black African population[2,23]. While in the developed countries, HCC is rare before the age of 40 irrespective of the HBV status, in underdeveloped countries, there is a distinct shift toward a younger age[2,23,24]. A study from China on Han Chinese population characterized by high prevalence of HBV infection demonstrated that polymorphism of GRP78 gene (genotypes AA and AG of rs430397) is associated with the development and prognosis of HCC[25].
HBV-induced carcinogenesis is essentially an inflammatory process resulting from the reaction of the host’s immune response to the presence of the virus. Integration of HBV DNA into host DNA is considered a critical step in HBV related HCC[26,27]. This leads to series of changes like cell cycle progression, inactivation of negative growth regulators, inhibition of the expression of p53 tumor suppressor gene and other tumor suppressor genes[26,27].
Recently, a striking increase in the incidence of obesity was recorded parallel to the increase in the incidence of HCC in several developed countries[28,29]. The increase in the number of HCC related cancer deaths in the United States has been documented while at the same time it is estimated that 25% of the population meet the diagnostic criteria for the metabolic syndrome[30]. In the great majority of the obese patients, the obesity is attributed to the metabolic syndrome. A recent meta-analysis demonstrated that the relative risk for HCC is 1.17 (95%CI: 1.02-1.34) in those who were overweight [body mass index (BMI) 25-30 kg/m2] and 1.89 (95%CI: 1.51-2.36) in those who were obese (BMI > 30 kg/m2)[31]. The incidence of the metabolic syndrome continues to increase in developed countries whereas the highest incidence is believed to occur in the United Kingdom (34% of the adult population)[32]. While obesity is present in up to 100% of patients with non-alcoholic fatty liver disease (NAFLD), the risk of liver steatosis is much higher in obese than in non-obese patients[5,30]. Finally, patients with liver steatosis are at high risk for developing cirrhosis and HCC[33]. Although NAFLD is currently the most common liver disease in developed countries, the incidence of HCC associated with NAFLD is lower than HCC associated with non-alcoholic steatohepatitis (NASH) (4%-27%)[33,34]. Today, the risk of HCC developing in NASH-cirrhotic patients challenges the risk of HCC developing in HCV-cirrhotic patients[35].
The pathogenesis linking obesity, NAFLD, NASH and HCC is still a subject of research. The relationship between obesity and HCC are thought to be mediated by factors associated to metabolic syndrome, NAFLD and NASH[17]. There is growing evidence that links obesity to chronic liver inflammation[17]. Moreover it is found that an excessive accumulation of fatty acids and glucose lead to increased expression of tumor necrosis factor-α, nuclear factor-kappa B, EGF heading to hepatic inflammation[36,37].
One other finding is that adipose tissue induces expression of leptin, a hormone that regulates body mass[38]. In animal models it was shown that leptin promotes angiogenesis and mediate the progression of NASH to HCC[38]. Leptin is found to upregulate JAK/STAT, AKT and ERK, i.e., signal transduction pathways involved in cancer progression in HCC cells[39].
Moreover, leptin levels are increased in patients with NASH, what may explain an increased vascular invasiveness in HCC patients with metabolic syndrome[40].
Aflatoxins is another risk factor for HCC. These toxins are metabolites of the widely distributed fungi Aspergillus flavus and Aspergillus parasiticus and their toxic, teratogenic, mutagenic and carcinogenic properties pose a serious risk to humans[41-43]. Approximately 4.5 to 5.5 billion people worldwide are at risk of exposure dominantly in Sub-Saharan Africa, Eastern Asia, and parts of South America[41,43]. Contamination occurs either in tropical and subtropical climates or in conditions where food drying and storage facilities are suboptimal. Aflatoxins are responsible for between 4.6% and 28.2% of all HCC cases worldwide[43]. The AFB1 toxin is metabolized in the liver by p450 enzymes forming AFB1-8,9-exo-epoxide, which further react with the p53 tumor suppressor gene[44,45]. Mutation at codon 249 of the p53 tumor suppressor gene accounts for 90% of p53 mutations in AFB1-related HCC[46]. There is a direct correlation between the degree of exposure to AFB1 and the incidence of HCC[42].
The study from Yu et al[47] found a synergistic effect of AFB1 and HBV in causing HCC since population with HBV who lived in the region of high exposure to AFB1 were associated to a mortality rate ten times higher than that of population with HBV living in the region of low exposure to the toxin.
Alcohol abuse, lasting more than 10 years, increases the chance for HCC development approximately five fold[48]. It is most common in the Americas (32% of HCC cases in the United States)[48] and Western Europe (45% of the cases in Italy[49]) and the incidence is increasing in Asia[7,9]. In principle, patients who develop the tumor have alcohol-induced cirrhosis[50].
Other less frequent risk factors include iron overload[51], hereditary hemochromatosis[52], tobacco smoking[53,54] and membranous obstruction of inferior vena cava[55].
Today, the diagnosis of HCC is established within the national surveillance programs in developed countries while the diagnosis of symptomatic, advanced stage, disease still remains the characteristic of underdeveloped countries. According to the American Association for the Study of Liver Diseases (AASLD) screening for HCC is recommended according to existing guidelines in all cirrhotic patients using ultrasound every six months[56]. Screening for chronic HBV carriers is recommended as well[57].
When a nodule is detected in a cirrhotic liver, a contrast-enhanced diagnostic procedure is strongly recommended. It is important to search for the typical signs of HCC (arterial phase enhancement and portal venous phase washout)[56]. The updated guidelines of AASLD consider that a non-invasive diagnosis of HCC can be established if a lesion > 10 mm has a typical vascular enhancement pattern in 4-phase multi-detector row CT (MDCT) or dynamic contrast enhanced magnetic resonance imaging (DCE-MRI)[56]. These guidelines were also accepted by the European societies[58].
Although MDCT is currently the most common imaging modality for detecting HCC, it is suboptimal for nodule characterization. DCE-MRI, with liver-specific contrast agents, has emerged as the preferred diagnostic modality for the investigation of HCC as it facilitates liver cancer characterization[59-61]. A recent meta-analysis[62] estimated the accuracy of MRI with liver-specific contrast agents compared to MDCT for the detection and characterization of HCC and demonstrated the superiority of MRI for the detection of HCC lesions < 20 mm.
For nodules smaller than 1 cm, a repeated ultrasound examination in three months intervals is recommended[56]. A biopsy is required only if imaging is inconclusive for lesions smaller than 2 cm, or it is atypical for lesions larger than 2 cm when the AFP level is not elevated[56]. However, biopsy carries an approximately 2% risk of tumor seeding[63] and the false-negative rate can be greater than 10% for small lesions[64]. The AASLD guideline has been prospectively validated for focal lesions 0.5 to 2.0 cm in size using MRI and contrast-enhanced ultrasound, and demonstrated a low sensitivity (33%) but a very high specificity (100%) for the diagnosis of HCC[65].
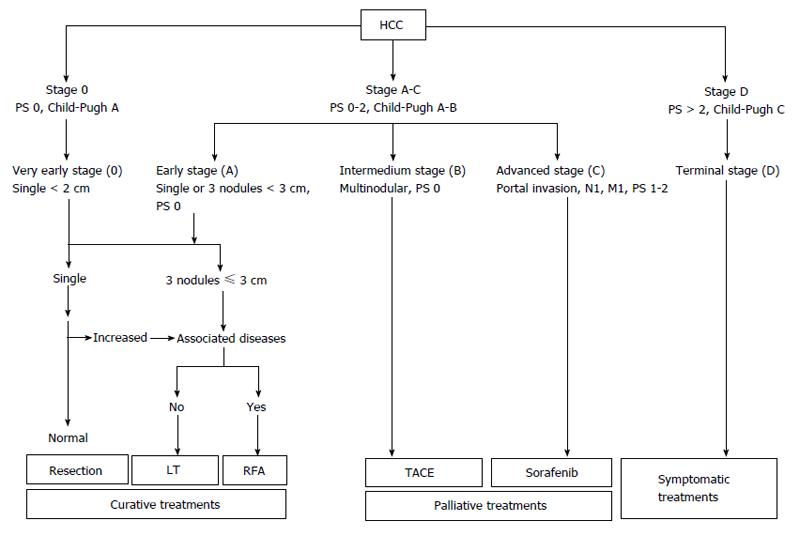
Since 1984 nine different staging systems have been developed and evaluated. The Barcelona-Clinic Liver Cancer (BCLC) staging system has emerged as the most useful to guide treatment decisions (Figure 1). BCLC is based on the analysis of independent studies in different clinical settings. It includes prognostic variables related to tumor status, liver functional status, and health performance status, together with treatment-dependent variables obtained from cohort studies and randomized clinical trials. The system links tumor stage with the treatment strategy allowing an estimation of life expectancy associated to specific HCC management[66].
BCLC demonstrated the best independent predictive power in many trials[67-71] when the entire patient population was included [not limited to patient population treated by surgery, radiofrequency ablation (RFA) or transarterial chemoembolization (TACE) only]. The BCLC staging system was externally validated[67,68,71,72] and has practically become an universal staging and treatment system. Moreover it was endorsed by European Association for the study of the liver (EASL) and AASLD as standard for patients with HCC[56,66].
However, other trials have demonstrated conflicting results thus favoring other staging systems[73-77]. Graf et al[78] have shown many limitations for the BCLC staging system (Table 1). Furthermore, as indicated by Maida et al[79] the BCLC staging system was not derived from a cohort of HCC patients by a multivariate analysis, and therefore it is not a prognostic model able to predict the mortality of HCC patients. Moreover, the intermediate stage (BCLC B) includes an extremely heterogeneous population in terms of both liver function and tumor characteristics and the main limitation of the BCLC is represented by its rigidity when it is acting as a treatment algorithm[79].
| No | BCLC classification system |
| 1 | Does not consider nodule location, which is essential for defining respectability |
| 2 | Does not respect etiology of cirrhosis |
| 3 | Is based on variables measured at diagnosis, which might change over time |
| 4 | Does not consider the possibility of liver transplantation for patients with Child C cirrhosis with hccs within the Milan criteria |
| 5 | Does not reflect contraindications of TACE |
| 6 | Recommends liver resection to single nodules only in absence of portal hypertension in very early (BCLC 0) and early stage (BCLC A), however probably portal hypertension might not affect survival in resected patients |
| 7 | Recommends liver resection in very early (BCLC 0) and early stage (BCLC A), however in selected patients hepatic resection is associated with good survival even in more advanced BCLC stages |
| 8 | Does not consider treatment sequences or combination therapies |
| 9 | Includes a very heterogeneous population in the intermediate stage (BCLC B) in respect to tumor burden and liver function |
| 10 | Does not consider other therapies than sorafenib in selected patients with advanced stage C with performance status 1 |
| 11 | Is not favorable as classification system in non-cirrhotic patients |
Importantly, treatment allocation should be decided by a multidisciplinary board based on individualized rather than on a guideline-based approach[80].
Although BCLC is the most comprehensive staging system, as it integrates tumor status, liver function and the performance status neither BCLC nor any other of the staging systems has been universally accepted, as pointed out by the AASLD guidelines[56], meaning that international consensus on the use of any given model is lacking.
Treatment allocation should be decided by a multidisciplinary board involving hepatologists, pathologists, radiologists, liver surgeons and oncologists guided by personalized-based medicine by. This approach is important not only to balance between different oncologic treatments strategies but also due to the complexity of the disease (combination chronic liver disease and the cancer) and due to the large number of potentially efficient therapies. When considering different treatment options the following is important: (1) there is a marked difference in available treatment modalities from one country to another; (2) historic studies are lacking, i.e., the results of potentially curative treatment modalities have never been compared to no treatment - today such studies are unethical; (3) the level of evidence for certain treatment modalities is limited to cohort studies and only a few randomized controlled trials; and (4) large, robust studies comparing results of different treatment modalities offered to patients in early stage disease are lacking as well.
Surgical treatment of HCC is established as a potentially curative treatment modality and includes liver transplantation, liver resection for HCC in cirrhotic livers and liver resection for HCC in non-cirrhotic livers.
Liver transplantation (LT) is the best treatment option as it removes both the tumor and the diseased liver parenchyma[81,82]. This is primarily important for patients with a Child-Pugh (CP) score C as it is the treatment of liver failure. The patient’s age (typically younger than 70 years), co-morbidities (e.g., cardiopulmonary disease, smoking, diabetes or renal disease), nutritional state (e.g., poor nutrition or morbidly obese), and social factors (e.g., adequate support, compliance, abstinence from alcohol and completion of an appropriate rehabilitation program) are all factors determining the patients’ eligibility for LT[81].
The most appropriate candidates for LT are patients that fit into the Milan criteria (a single tumor < 5 cm or up to 3 tumors of < 3 cm) achieving a 5-year survival rate of 70%-80%. In these patients the recurrence rates are approximately 10%[83,84].
The Milan criteria can be expanded to include more patients primarily by liberalizing the restrictions on tumor size. Yao et al[85] demonstrated that using the University of California San Francisco criteria (single nodule < 6.5 cm or ≤ 3 nodules each ≤ 4.5 cm, with total combined tumor diameter ≤ 8 cm), a 75% 5-year survival rate is achievable. Kaido et al[86] reported that using the Kyoto criteria (a combination of tumor number ≤ 10, maximal diameter of each tumor ≤ 5 cm, and serum des-γ-carboxy prothrombin levels ≤ 400 mAU/mL), the 5-year survival rate after living donor LT is 82%.
Mazzaferro et al[87] have proposed the “Metro ticket price” concept - the further one goes in expanding the criteria for LT, the more one “pays”, i.e., the more you deviate from the Milan criteria, the survival rate decreases and recurrence rate increases.
Due to the limited number of donors and the scarcity of sufficient available data, current guidelines do not recommend LT for HCC patients outside the Milan criteria[58,88]. Patients with a compromised liver function (CP - B or C) should be listed for LT while allocation of this treatment modality to CP class A patients instead of surgical resection is still an area of debate. The Barcelona Clinic has analyzed their results for surgical resection and LT in an intent-to-treat manner, although the patients were never compared directly in a randomized trial[89]. The five-year survival rates for resection and LT were nearly identical if patients for resection were carefully selected (CP class A, normal bilirubin levels and no portal hypertension).
Waiting time for LT is a serious obstacle in many national transplant programs worldwide. When the waiting list for LT is longer than 12 mo the drop-out rates can reach 25% of HCC patients listed for LT[90,91]. Clearly, if patients with more advanced tumors are included as a result of expanded listing criteria the dropout rate will be higher and this will lead to poor survival figures. In that regard the potential benefit of TACE, TARE, RFA and others, applied in the neoadjuvant setting include “bridging” or “down-staging” strategies to increase the number of HCC patients qualifying for LT[92].
Furthermore another important concept of LT is salvage LT that saves the donor pool and can effectively be performed for patients with recurrence or liver function deterioration following resection for HCC. This does not increase the perioperative mortality and has similar long-term survival compared to primary LT[93].
Liver transplantation can also be offered to patients with non-resectable HCC in normal livers providing 5-year survival rates of 59%[94]. In contrast to LT for HCC in cirrhosis the tumor size is not a predictor of post-transplant survival[94].
Finally, many controversies related to LT were confronted during an international consensus conference held in 2010, in Switzerland that resulted in 37 statements and recommendations[95]. These recommendations reflect the current state of scientific evidence regarding the LT and reflect differences in clinical practice of LT between continents, countries and institutions. In each controversial topic the strength of recommendation was conditioned by the level of evidence that was in the majority of instances 2 or less reflecting the quality of evidence that is currently available. Among the 37 recommendations only 17 are strong (presented in Table 2) while the others are week or their strength could not be established due to insufficient data[95].
| Assessment of candidates with HCC for liver transplantation |
| When considering treatment options for patients with HCC, the BCLC staging system is the preferred staging system to assess the prognosis of patients with HCC |
| The TNM system (7th ed) including pathological examination of the explanted liver, should be used for determining prognosis after transplantation with the addition of assessment of microvascular invasion |
| Either dynamic CT or dynamic MRI with the presence of arterial enhancement followed by washout on portal venous or delayed imaging is the best non-invasive test to make a diagnosis in cirrhotic patients suspected of having HCC and for preoperative staging |
| Extrahepatic staging should include CT of the chest, and CT or MRI of the abdomen and pelvis |
| For patients with lesions smaller or equal to 10 mm, non-invasive imaging does not allow an accurate diagnosis and should not be used to make a decision for or against transplantation |
| Criteria for listing candidates with HCC in cirrhotic livers for deceased donor LT |
| Preoperative assessment of the size of the largest tumor or total diameter of tumors should be the main consideration in selecting patients with HCC for liver transplantation |
| The Milan criteria are currently the benchmark for the selection of HCC patients for liver transplantation, and the basis for comparison with other suggested criteria |
| Biomarkers other than α-fetoprotein cannot yet be used for clinical decision making regarding liver transplantation for HCC |
| Indication for liver transplantation in HCC should not rely on microvascular invasion because it cannot be reliably detected prior to transplantation |
| Role of down-staging |
| Liver transplantation after successful down-staging should achieve a 5-yr survival comparable to that of HCC patients who meet the criteria for liver transplantation without requiring down-staging |
| Criteria for successful down-staging should include tumour size and number of viable tumours |
| Managing patients of the waiting list |
| Periodic waiting-list monitoring should be performed by imaging (dynamic CT, dynamic MRI, or contrast-enhanced US) and α-fetoprotein measurements |
| Patients found to have progressed beyond criteria acceptable for listing for liver transplantation should be placed on hold and considered for down-staging |
| Patients with progressive disease in whom locoregional intervention is not considered appropriate, or is ineffective, should be removed from the waiting list |
| Role of living donor LT |
| Living donor LT must be restricted to centers of excellence in liver surgery and liver transplantation to minimize donor risk and maximize recipient outcome |
| In patients following living donor LT for HCC outside the accepted regional criteria for deceased donor LT, re-transplantation for graft failure using a deceased donor organ is not recommended |
| Post-transplant management |
| Liver re-transplantation is not appropriate treatment for recurrent HCC |
The highest level of evidence and the strength of recommendation is related to the assessment of candidates for LT and in defining criteria for listing candidates with HCC in cirrhotic livers for deceased donor LT. In regard to HCC patients in non-cirrhotic liver LT this procedure may be considered as salvage transplantation for patients with intrahepatic recurrence following liver resection and no evidence of lymph node or macrovascular invasion[95].
The role of down-staging was evaluated in perspective of different loco-regional treatment options that are presented in the literature (TACE, TARE, RFA). Although the largest experience is linked to TACE and RFA, based on existing evidence, no recommendation can be made for selecting a specific loco-regional therapy for down-staging[95].
Living donor LT is an important alternative to deceased donor liver transplantation in the present circumstances of increasing number of HCC patients listed for LT. It is conducted in a limited number of centers worldwide. Although it facilitates access to LT, recent meta analysis demonstrated that living donor LT is associated with a higher rate of surgical complications following transplantation[96].
In that sense an important recommendation is derived from the consensus conference, i.e., that living donor LT must be restricted to centers of excellence in liver surgery and liver transplantation to minimize donor risk and maximize recipient outcome[95].
During the past decade a tremendous improvement in the understanding of liver anatomy, advances in technology, anesthesiology and postoperative intensive care and the application of intraoperative ultrasonography have established surgical resection as a widely accepted first-line curative treatment option for HCC patients. Surgical resection for HCC is a safe and reliable procedure and, unlike LT, it is available in many countries and institutions. Presently, when considering liver resection, the main focus has shifted from the tumor towards the functional capacity of the remnant liver.
Liver resection for HCC is considered in two different settings. One is liver resection for HCC in non-cirrhotic, “normal” livers and the other is liver resection in cirrhotic livers, when special attention is attributed to the functional capacity of the remnant liver. Considering the improvement in the technical feasibility of complex liver resection there are practically no more non-resectable tumors, but considering the functional capacity of the remnant liver only a relatively small percentage of HCC patients with cirrhotic livers can be offered curative-intent liver resection.
Patients with HCC in non-cirrhotic livers are rare in the western world; only 5%-15% of HCC patients have a normal, non-cirrhotic parenchyma[58,97,98]. They are diagnosed late with large-size tumors sometimes with major vascular invasion. Liver resection is the only curative treatment in these patients and up to 70%-80% of functional liver parenchyma can be removed[78].
The 5-year disease free survival of non-cirrhotic HCC patients managed by liver resection is around 50% depending on resection status, UICC stage, vascular invasion, tumor size > 10 cm and tumor grading[98-101]. About 50% of these patients will have recurrence within 2 years after curative resection[58,101]. Repeated liver resection is the treatment of choice for patients with intrahepatic recurrence having a similar prognostic outcome as the primary resection[102].
According to the BCLC staging system, surgical resection for HCC in cirrhosis is reserved for patients in the BCLC 0 stage (single tumor < 2 cm, Child A, ECOG 0 without portal hypertension and normal bilirubin level) and it is feasible in selected patients in the BCLC A stage. However, clinical practice worldwide (not only in Japan) is not limited to the frame recommended by the BCLC staging. Moreover, it is expanded even to selected patients belonging to BCLC intermediate stage B group. This has to be considered within a context that in many developing countries screening and surveillance programs are lacking, therefore the majority of patients are diagnosed in an advanced stage of the disease when surgical resection is still feasible[103,104]. Strict adherence to the BCLC staging system would direct the majority of patients to palliative treatment only.
Despite of recent advances in surgical techniques and perioperative care, liver resection is challenged by the poor functional reserve of the cirrhotic liver, the impaired regeneration capacity, elevated portal venous pressure, and other co-morbidities of the HCC patients[105,106]. Although reserved for high-volume centers, liver resection is justified even for patiens with large and multinodular HCC[107-109].
A study from Ishizawa et al[110] has demonstrated that neither multiple tumors nor portal hypertension are surgical contraindications for HCC. Two other studies have verified that liver resection is feasible even in Child B patients and in selected patients a major liver resection is feasible as well[111,112]. According to Ho et al[113] liver resection is associated with better overall survival comparing to TACE (37.9 mo vs 17.3 mo) even for patients with multinodular HCC. In patients with large tumors, TACE is associated with low response rate and a modest 3 years survival rate[108,109].
Several studies confirmed that blood loss has a negative impact on the perioperative morbidity, mortality and long-term outcome[114,115] therefore a control of bleeding is mandatory when performing liver resection. Vascular occlusion techniques[116] are effective in reducing blood loss, but it was found that they compromise hepatic functional reserve in conditions of a preexistent liver disease[117,118]. Fu et al[119] found an earlier recovery of the postoperative liver function after hemihepatic vascular inflow occlusion compared with the Pringle maneuver, however it is technically more demanding and potentially associated with more bleeding in cirrhotic livers.
Prediction of the future, functional remnant liver volume (FLR) is crucial for postoperative morbidity and mortality. A remnant volume of at least 40% should remain following resection of cirrhotic livers in order to preserve adequate liver function[120]. Three dimensional measurements of liver volumes based on MDCT or magnetic resonance imaging (MRI) and more important post-processing software are important for predicting the FLR after liver resection.
Portal vein embolization (PVE) has an important role as an effective tool in inducing hypertrophy of the non-embolized hepatic segments. An increase of the FLR volume of 20%-46% can be achieved after 2-8 wk[121,122]. When the FLR volume is insufficient PVE is considered an important therapeutic step before extended resection. Recently, one other approach has been described for increasing the FLR volume in a two-stage procedure for patients undergoing extended liver resection. In situ liver transection combined with portal vein ligation emerged as a procedure associated with rapid growth of the FLR[123,124] and was tested in the settings of HCC in cirrhotic livers[125] even in conditions of major vascular invasion[126]. The median FLR volume increase was 18.7% within one week after the first step and 38.6% after the second step[125]. More studies are needed before the real merits of ALPPS can be evaluated.
The use of metabolic tests, namely the indocyanine green test is another tool to assess the liver functional capacity in order to avoid postoperative liver failure[127]. As indicated in two surveys[120,128] it is widely used in Asia and the indocyanine green retention rate at 15 min (ICGR-15) is integrated into the decision tree for deciding the safe limit of hepatectomy[127]. In the western world the ICGR-15 test is used in a limited number of centers and in selective cases only[120]. In HCC patients with cirrhotic livers characterized by normal bilirubin level and absence of ascites the ICGR-15 is the main determinant for performing a liver resection[127].
The anatomic liver resection should be associated with improved outcome as HCC tumors have a tendency for local portal vein invasion with possible extension toward the main portal vein. However conflicting results are present in the literature. Two studies[129,130], demonstrated that anatomic resection is an independent predictor of improved recurrence-free survival and it significantly improves the disease-free survival rates. Anatomic resection is recommended in the EASL guidelines as the preferred approach if sufficient remnant liver volume can be preserved[56]. The use of dye widely practiced in Japan may aid delineation of tumor bearing segments and facilitate complete anatomical resection[131,132].
Laparoscopic liver resection for HCC in cirrhotic livers is an established and safe procedure performed in many centers worldwide[128]. There are no randomized controlled trials that has compared laparoscopic vs open liver resection in HCC patients. Four meta-analyses[133-136] of nonrandomized studies found that laparoscopic resection was associated with significantly less blood loss, lower transfusion requirements, lower overall morbidity, and shorter length of hospital stay without a significant difference in length of operation, surgical margin status, or tumor recurrence rates.
Tumor ablation can be achieved by chemical (ethanol, acetic acid) or thermal [radiofrequency ablation-RFA, microwave ablation (MWA)] ablation and it is the treatment of choice in patients with single, small tumors who are not candidates for surgery. According to the BCLC staging and treatment algorithm these patients are classified as BCLC A patients[58]. BCLC 0 patients may also be managed by this treatment modality, although the algorithm primarily allocates resection to this group of patients[58]. When procedure limitations are strictly respected (tumor size, tumor location, duration of the treatment, maintaining the required temperature in the tumor zone, etc.) tumor ablation is a curative treatment option for the management of carefully selected HCC patients.
Historically, tumor ablation started as chemical ablation using percutaneous ethanol injection (PEI) for the management of nodular-type HCC. There is considerable experience with PEI since it is an established technique. PEI induces coagulation necrosis of the tumor as a result of cellular dehydration, protein denaturation, and chemical occlusion of small tumor vessels[137]. Several studies confirm that tumors < 2 cm can be successfully treated by PEI achieving equivalent results to thermal ablation techniques[137-139]. For larger tumors PEI is inferior to thermal ablation and therefore should not be performed[138-142]. However, PEI should not be neglected and can be used in underdeveloped regions as a very useful treatment modality.
Thermal ablation has now largely replaced PEI, initially with RFA and recently with MWA[137,139]. Although it is an interventional procedure performed percutaneously by interventional radiologists or jointly by an interventional radiologist and liver surgeon, a multidisciplinary approach which provides important advantages, as described by Poon et al[143]. Thermal ablation can also be done via an open or laparoscopic surgical approach.
The main advantage of thermal ablation is related to its low major morbidity (2.2%-3.1%) and mortality (0.1%-0.5%) rates[144,145]. Major complications include intraperitoneal hemorrhage, hepatic abscess, bile duct injury, and liver decompensation[56,144,145]. Tumor seeding along the needle track has been reported as a rare (0.5%) late complication of RFA[146].
The most important observations resulted from explants studies following LT and demonstrated complete tumor necrosis in explanted liver specimens in 83% of tumors > 3 cm and in 88% of tumors in non-perivascular locations[54,56,144,145]. Clearly the efficacy of RFA is reduced with increasing tumor size and the presence of large vessels[147]. RFA should be applied for tumors less than 3 cm in size, bearing in mind that success is related to the total volume of the tumor tissue that has to be ablated.
Lencioni et al[145,148] have demonstrated 61% 5-year survival in patients with Child A cirrhosis and solitary HCC, compared with 51% in patients with Child A cirrhosis and multiple tumors and 31% in patients with Child B cirrhosis. Livraghi et al[149] has reported complete tumor response in 97% of tumors ≤ 2 cm, with 5-year survival in patients with preserved hepatic function of 68%, challenging resection as the first-line approach in such cases.
Hasegawa et al[150] concluded that resection was associated with a higher overall survival and lower recurrence rate than RFA or PEI in the treatment of HCC ≤ 3 cm.
A challenging question is whether emerging alternative, MWA, will replace RFA. Compared to RFA, MWA is less-susceptible to the heat sink effect of nearby blood vessels and produces a larger zone of necrosis[151].
In a non-randomized study published in 2013[152] that investigated the therapeutic efficacy of percutaneous RFA and MWA for HCC < 5 cm no significant differences were found between the two procedures in the percentage of complete ablation local tumor progression, distant recurrence and overall survival. Clearly, more studies are needed to compare the two ablation techniques.
According to the BCLC staging and treatment algorithm TACE is indicated for patients classified as BCLC B stage, that is an intermediate stage composed of a very heterogeneous patient population[56,153,154]. A Cochrane review[154] clearly confirmed the survival benefit of this treatment modality. However, TACE is not standardized in regard to: (1) the procedure technique; (2) the choice of embolic agent; (3) the choice of applied medications; and (4) the schedule (on demand or at fixed intervals). In clinical practice TACE is performed by injection of chemotherapy with or without lipiodol, followed by the injection of embolic particles. This procedure is considered as conventional TACE. Innovative step forward was the development of drug-eluting beads (DC Bead) used to increase tumor drug delivery. However, the PRECISION V study[155] designed to compare the two TACE procedures failed to demonstrate a clear superiority of DC Bead-TACE (one-sided P = 0.11). The difference between the two TACE procedures was found in the complete response, objective response, and disease control favoring DC Bead group (27% vs 22%, 52% vs 44%, and 63% vs 52%, respectively)[155].
Complications of TACE include non-target embolization, the post embolization syndrome (fever, abdominal pain, ileus), liver failure, cholecystitis and acute portal vein thrombosis[154]. The procedure-related mortality is less than 5% which defines TACE as a safe procedure[154]. Main portal vein thrombosis, poor liver function, and extrahepatic spread have been shown to be predictors of poor outcome and are considered contraindications for chemoembolization[154].
Several aspects of TACE treatment require special consideration. In clinical practice an attempt should be made to achieve the supraselective approach (STACE) using micro-catheters in order to deliver chemotherapy as close as possible to the tumor site. Unfortunately this aspect was not much elaborated in clinical trials. Only one trial[156] on 60 patients who were candidates for LT, found STACE to be associated with complete tumor necrosis in a larger proportion of patients (30.8% vs 6.9%, P = 0.02) compared to selective TACE group. Still, a 5-year disease-free survival was similar in both groups (76.8% vs 74.8%)[156]. In conclusion, there is no clear relationship between the therapy-induced complete necrosis and long-term survival.
The combination of TACE and RFA is another challenging treatment option practiced in many centers worldwide. Recent meta-analyses[157] showed that the combination of RFA and TACE was associated with a significantly higher overall survival rates (OR 1 year = 2.39, 95%CI: 1.35-4.21, P = 0.003; OR 3 years = 1.85, 95%CI: 1.26-2.71, P = 0.002), and recurrence-free survival rate (OR 1 year = 2.00, 95%CI: 1.26-3.18, P = 0.003; OR 3 years = 2.13, 95%CI: 1.41-3.20, P < 0.001) compared with RFA alone[157]. The quality of the evidence was high for the 1- and 3-year survival[157].
Two recent meta-analysis[158,159] elaborated the combination of TACE and sorafenib and found evident improvement in the objective response, time to progression and overall survival although the sorafenib associated AEs were more frequent in the combination therapy group[158,159].
Another important meta-analysis[160] examined the efficacy of TACE for HCC patients with portal vein thrombosis (PVTT) and found that TACE improves the 1-year survival of patients with HCC and PVTT. As current treatment algorithms contraindicate TACE in patients with main trunk PVTT more trials are required to confirm these findings[54,56,66].
As already indicated the BCLC B stage (an intermediate stage) is composed of a very heterogeneous patient population. Several studies confirmed limited treatment efficacy of TACE for HCC patients with large multinodular tumors[161,162]. The objective response to TACE treatment is found in 52% of patients (PRECISION V trial[155]) leaving large proportion of patients without an effective treatment. It is important to note that TACE is contraindicated in patients with main trunk PVTT. These findings led to development of new therapies for optimal management of these subcategories of HCC patients belonging to BCLC B stage[163].
Radio-embolization via hepatic artery using microspheres impregnated with yttrium-90 (TARE) is a new emerging treatment option that is available in a limited number of centers worldwide[162,163]. TARE uses the same concept as TACE in regards the technical aspect of the procedure. The difference is reflected in the mode of action. In TARE the embolic particles (microspheres) are 3-10 times smaller than those used in TACE (25-35 micron in diameter)[164]. Yttrium-90 (beta emitter with a short half-life) microspheres are used to produce tumor necrosis by internal delivery of tumoricidal dose of radiation directly to the tumor with nearly no embolic effect on the vessels[165]. The safety and efficacy of TARE is well established in many trials[164,166-168] and post-embolization syndrome is found in 20%-55% of cases[164,166]. TARE was found to be a safe procedure in HCC patients with PVTT[169,170]. Initially, TARE was indicated in HCC patients who progressed or relapsed after the TACE treatment or in HCC patients not amenable to TACE (large multinodular tumors or presence of PVTT)[164]. Although Sangro et al[162] found survival benefit for TARE comparing to TACE as a first-line treatment other studies reported no significant difference in survival[168,171,172]. Potential advantage of TARE over TACE can be attributed to early-stage HCC patients listed for LT who are candidates for bridging or down-staging therapy[173,174].
After years of disappointment with the results of numerous trials testing the efficacy of different drugs in the medical management of HCC, two milestone studies[175,176] have established sorafenib as a treatment of choice for BCLC C patients according to the EASL-EORTC guidelines[56].
Sorafenib is a molecular inhibitor of several tyrosine protein kinases (VEGFR and PDGFR); Raf kinases (C-Raf than B-Raf)[177,178] and intracellular serine/threonine kinases (C-Raf, wild-type B-Raf and mutant B-Raf)[179] Sorafenib treatment induces autophagy[180], which suppresses tumor growth.
Although sorafenib was introduced as well-tolerated drug a subanalysis of the two leading[175,176] and other[181,182] studies have shown that the tolerability of sorafenib was suboptimal[183]; it was down-dosed in more than 50% and interrupted in 45% of patients due to severe adverse events or compromised liver function[183].
Therefore the most important side effects are gastrointestinal[184] (diarrhea 43%, increased lipase 41%, increased amylase 30%, nausea 23%, anorexia 16%, vomiting 16%, and constipation 15%), dermatologic[185] (rash/desquamation 40%, hand-foot skin reaction 30%, alopecia 27%, pruritus 19%, and dry skin 11%), cardiovascular[186]. (Hypertension 17%, angioedema, and congestive heart failure), hematologic[187]. (Hypoalbuminemia 49%, hemorrhage 15%, anemia and thrombocytopenia) and nervous system side effects[186] (neuropathy 13% and headache 10%).
One of the two milestone studies (SHARP/phase III)[175] conducted in the western world have shown that sorafenib prolonged median survival from 7.9 mo (placebo group) to 10.7 mo (sorafenib group) (HR = 0.69; 95%CI: 0.55-0.87; P = 0.00058). Sorafenib also improved the time to progression (from 2.8 mo to 5.5 mo). Another milestone study conducted in Asia confirmed the outcomes of the SHARP trial, i.e., a phase III Asia-Pacific trial[176] have shown a median overall survival of 6.5 mo (treatment group) comparing to 4.2 mo (placebo group) (HR = 0.68; 95%CI: 0.50-0.93; P = 0.014).
Another important study was a phase IV, GIDEON trial[188], conducted with the aim to evaluate the safety of sorafenib treatment in HCC patients in real-life conditions. In 2011 the second interim analysis showed a median survival of 10.3 mo for Child A patients and 4.8 mo for Child B patients. The amount of adverse events was comparable to the two milestone studies.
The use of sorafenib in adjuvant settings was addressed in the STORM trial. In mid 2014 major pharmaceutical companies Bayer and Onyx announced that the STORM trial did not meet its primary endpoint. During the ASCO annual meeting in 2014 Bruix et al[189] reported that both primary and secondary endpoints were not met. The trial enrolled the largest cohort of patients with HCC treated in this setting. Overall, 1114 patients were equitably randomized to take either sorafenib or placebo. The study did not met its primary and secondary endpoints since no differences were observed regarding recurrence-free survival (33.4 mo vs 33.8 mo; HR = 0.94; 95%CI: 0.78-1.13, P = 0.26), time to recurrence (38.6 mo vs 35.8 mo; HR = 0.89, 95%CI: 0.73-1.08) and overall survival (not reached vs not reached, HR = 0.99, 95%CI: 0.76-1.30, P = 0.48). In this trial, a higher rate of sorafenib discontinuation due to drug-adverse events was observed compared to placebo (24% vs 7%)[190].
Additional studies have evaluated other targeted agents either in combination with sorafenib, or designed as head-to-head compared to sorafenib, or as second-line treatments following disease progression or inability to tolerate sorafenib; however, all these trials failed to demonstrate an improvement in overall survival[190].
HCC is a difficult to treat and extremely complex malignant disease. Epidemiological data confirms an increasing number of new cases each year and this rise will persist due to the burden linked to HCV and obesity. Since the number of new HCC cases being diagnosed each year is nearly equal to the number of deaths from this cancer it is clear that the international scientific community and healthcare systems worldwide have no efficient answer to HCC. There are marked differences between countries in providing disparate quality of healthcare considering screening and surveillance programs; available treatment modalities and drugs; reimbursement of specific treatment options by the state-funded health insurance. In many countries worldwide liver transplantation is not a therapeutic option. In countries with national LT program the donor pool is a serious obstacle for treating more patients. Surveillance programs, so essential for the diagnosing an early stage HCC, are lacking in many countries. The experience from Japan clearly confirms importance of a successful surveillance program. Liver resection and TACE are the two treatment modalities offered to HCC patients even in underdeveloped countries. Since treatment allocation should be decided by a multidisciplinary board involving hepatologists, pathologists, radiologists, liver surgeons and oncologists guided by individualized-based medicine, HCC patients should be managed in high-volume, tertiary, university centers. This approach is important to achieve the best possible outcome from a variety of potentially useful therapies and for research purposes.
Different combination therapies tested in various studies failed to demonstrate a real benefit in terms of overall survival. This is mainly due to the complexity of the disease and due to the extremely heterogeneous patient populations included in clinical trials. The consensus conference on LT for HCC has shown that many controversies remained unanswered due to the lack of evidence. Therefore, high-quality randomized trials with better patient stratification are mandatory in the future to find patient populations that can benefit from certain treatment modalities. Basic research in HCC carcinogenesis is equally important. Combinations of different treatment modalities should be more exploited in order to improve survival and the quality of life of HCC patients.
In the management of HCC patients, several recommendations are important: (1) to establish a national surveillance program in as many countries as possible; (2) to further improve treatment modalities for patients on the waiting list for LT; (3) to improve the safety of liver resection and to reduce the recurrence rates following resection; (4) to investigate further and to upgrade results of the TACE treatment modality; (5) to continue research on novel molecular therapies; and (6) to continue research on novel molecular markers for better patient selection for various treatment modalities.
P- Reviewer: Baran B, Lai S, Zhu X S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Bailar JC, Gornik HL. Cancer undefeated. N Engl J Med. 1997;336:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 277] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2002;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13556] [Article Influence: 677.8] [Reference Citation Analysis (1)] |
| 3. | National Cancer Institute. Cancer Trends Progress Report - 2009/2010 Update. [Accessed 2015 Mar]. Available from: http://progressreport.cancer.gov/. |
| 4. | Mortality Database. WHO Statistical Information System. Geneva: World Health Organization 2008; Available from: http://www.who.int/whosis. |
| 5. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1325] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 6. | El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 879] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 8. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11832] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 9. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 10. | GLOBOCAN 2012: Estimated Cancer Incidence, Mortality And Prevalence Worldwide In 2012. Lyon: International Agency for Research on Cancer (IARC). [Accessed 2012]. Available from: http://www-dep.iarc.fr. |
| 11. | Kew MC. Hepatocellular carcinoma: epidemiology and risk factors. J Hepatocellular Carcinoma. 2014;1:115-125. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37 Suppl 2:S88-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 14. | Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol Res. 2015;45:59-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | van Meer S, de Man RA, Siersema PD, van Erpecum KJ. Surveillance for hepatocellular carcinoma in chronic liver disease: evidence and controversies. World J Gastroenterol. 2013;19:6744-6756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Harnois DM. Hepatitis C virus infection and the rising incidence of hepatocellular carcinoma. Mayo Clin Proc. 2012;87:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651-5661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 18. | Asia Pacific Cohort Studies Collaboration. The burden of overweight and obesity in the Asia-Pacific region. Obes Rev. 2007;8:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Kew M, François G, Lavanchy D, Margolis H, Van Damme P, Grob P, Hallauer J, Shouval D, Leroux-Roels G, Meheus A. Prevention of hepatitis C virus infection. J Viral Hepat. 2004;11:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, Romano C, Adinolfi LE. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 21. | Hutin Y, Kitler ME, Dore G. Global burden of disease for hepatitis C. J Clin Pharmacol. 2003;43:20-29. |
| 22. | Lemon SM, McGivern DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris). 2010;58:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Nguyen VT, Amin J, Law MG, Dore GJ. Predictors and survival in hepatitis B-related hepatocellular carcinoma in New South Wales, Australia. J Gastroenterol Hepatol. 2009;24:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Zhu X, Chen MS, Tian LW, Li DP, Xu PL, Lin MC, Xie D, Kung HF. Single nucleotide polymorphism of rs430397 in the fifth intron of GRP78 gene and clinical relevance of primary hepatocellular carcinoma in Han Chinese: risk and prognosis. Int J Cancer. 2009;125:1352-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981;305:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 497] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Kew MC. Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26 Suppl 1:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Calle EE, Rodriguez C, Walt-Thurmond K. Over-weight, obesity, and cancer risk. Lancet Oncol. 2002;348:625-1638. |
| 29. | Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 623] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 30. | Borena W, Strohmaier S, Lukanova A, Bjørge T, Lindkvist B, Hallmans G, Edlinger M, Stocks T, Nagel G, Manjer J. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 423] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 32. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2127] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 33. | Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 526] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 34. | Nobili V, Alisi A, Grimaldi C, Liccardo D, Francalanci P, Monti L, Castellano A, de Ville de Goyet J. Non-alcoholic fatty liver disease and hepatocellular carcinoma in a 7-year-old obese boy: coincidence or comorbidity? Pediatr Obes. 2014;9:e99-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, Boffetta P, Dahm CC, Overvad K, Tjønneland A. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. 2013;132:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;14:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5762] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 38. | Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, Lang T, Fukuda T, Yamashina S, Kitamura T. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 300] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497-2507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 387] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 40. | Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, Samarasinghe D, George J. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 237] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Wild CP, Pionneau FA, Montesano R, Mutiro CF, Chetsanga CJ. Aflatoxin detected in human breast milk by immunoassay. Int J Cancer. 1987;40:328-333. [PubMed] |
| 42. | Strosnider H, Azziz-Baumgartner E, Banziger M, Bhat RV, Breiman R, Brune MN, DeCock K, Dilley A, Groopman J, Hell K. Workgroup report: public health strategies for reducing aflatoxin exposure in developing countries. Environ Health Perspect. 2006;114:1898-1903. [PubMed] |
| 43. | Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur J Cancer. 2012;48:2125-2136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 44. | Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 968] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 45. | Kirk GD, Camus-Randon AM, Mendy M, Goedert JJ, Merle P, Trépo C, Bréchot C, Hainaut P, Montesano R. Ser-249 p53 mutations in plasma DNA of patients with hepatocellular carcinoma from The Gambia. J Natl Cancer Inst. 2000;92:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Gouas D, Shi H, Hainaut P. The aflatoxin-induced TP53 mutation at codon 249 (R249S): biomarker of exposure, early detection and target for therapy. Cancer Lett. 2009;286:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Yu MW, Lien JP, Chiu YH, Santella RM, Liaw YF, Chen CJ. Effect of aflatoxin metabolism and DNA adduct formation on hepatocellular carcinoma among chronic hepatitis B carriers in Taiwan. J Hepatol. 1997;27:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 396] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 49. | Chiesa R, Donato F, Tagger A, Favret M, Ribero ML, Nardi G, Gelatti U, Bucella E, Tomasi E, Portolani N. Etiology of hepatocellular carcinoma in Italian patients with and without cirrhosis. Cancer Epidemiol Biomarkers Prev. 2000;9:213-216. [PubMed] |
| 50. | Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma in cirrhotic and noncirrhotic livers. A clinico-histopathologic study of 804 North American patients. Am J Clin Pathol. 1996;105:65-75. [PubMed] |
| 51. | Nahon P, Ganne-Carrié N, Trinchet JC, Beaugrand M. Hepatic iron overload and risk of hepatocellular carcinoma in cirrhosis. Gastroenterol Clin Biol. 2010;34:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Fracanzani AL, Conte D, Fraquelli M, Taioli E, Mattioli M, Losco A, Fargion S. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology. 2001;33:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 54. | International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans. Lyon: IARC 2004; 1-1438. |
| 55. | Gwon D, Ko GY, Yoon HK, Sung KB, Kim JH, Lee SS, Lee JM, Ohm JY, Shin JH, Song HY. Hepatocellular carcinoma associated with membranous obstruction of the inferior vena cava: incidence, characteristics, and risk factors and clinical efficacy of TACE. Radiology. 2010;254:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 57. | Yang HI, Sherman M, Su J, Chen PJ, Liaw YF, Iloeje UH, Chen CJ. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Clin Oncol. 2010;28:2437-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 58. | European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 362] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 59. | Ba-Ssalamah A, Uffmann M, Saini S, Bastati N, Herold C, Schima W. Clinical value of MRI liver-specific contrast agents: a tailored examination for a confident non-invasive diagnosis of focal liver lesions. Eur Radiol. 2009;19:342-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 386] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 61. | Floriani I, D’Onofrio M, Rulli E, Chen MH, Li R, Musicco L. Performance of imaging modalities in the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ultraschall Med. 2013;34:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Chen L, Zhang L, Bao J, Zhang J, Li C, Xia Y, Huang X, Wang J. Comparison of MRI with liver-specific contrast agents and multidetector row CT for the detection of hepatocellular carcinoma: a meta-analysis of 15 direct comparative studies. Gut. 2013;62:1520-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 64. | Caturelli E, Solmi L, Anti M, Fusilli S, Roselli P, Andriulli A, Fornari F, Del Vecchio Blanco C, de Sio I. Ultrasound guided fine needle biopsy of early hepatocellular carcinoma complicating liver cirrhosis: a multicentre study. Gut. 2004;53:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 65. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 727] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 66. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 67. | Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 68. | Kim BK, Kim SU, Park JY, Kim do Y, Ahn SH, Park MS, Kim EH, Seong J, Lee do Y, Han KH. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naïve patients with hepatocellular carcinoma. Liver Int. 2012;32:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Cillo U, Bassanello M, Vitale A, Grigoletto FA, Burra P, Fagiuoli S, D’Amico F, Ciarleglio FA, Boccagni P, Brolese A. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol. 2004;40:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Pascual S, Zapater P, Such J, García-Herola A, Sempere L, Irurzun J, Palazón JM, Carnicer F, Pérez-Mateo M. Comparison of staging systems to predict survival in hepatocellular carcinoma. Liver Int. 2006;26:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 332] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 72. | Santambrogio R, Salceda J, Costa M, Kluger MD, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D. External validation of a simplified BCLC staging system for early hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee PC, Loong CC, Chiang JH, Huo TI, Lee SD. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010;116:3006-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, Enea M, Attanasio M, Galia M, Alessi N. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Lin CY, Kee KM, Wang JH, Lee CM, Chen CL, Changchien CS, Hu TH, Cheng YF, Hsu HC, Wang CC. Is the Cancer of the Liver Italian Program system an adequate weighting for survival of hepatocellular carcinoma? Evaluation of intrascore prognostic value among 36 subgroups. Liver Int. 2009;29:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Huo TI, Lin HC, Hsia CY, Wu JC, Lee PC, Chi CW, Lee SD. The model for end-stage liver disease based cancer staging systems are better prognostic models for hepatocellular carcinoma: a prospective sequential survey. Am J Gastroenterol. 2007;102:1920-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Chung H, Kudo M, Takahashi S, Hagiwara S, Sakaguchi Y, Inoue T, Minami Y, Ueshima K, Fukunaga T, Matsunaga T. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol. 2008;23:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Graf D, Vallböhmer D, Knoefel WT, Kröpil P, Antoch G, Sagir A, Häussinger D. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014;25:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Maida M, Orlando E, Cammà C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol. 2014;20:4141-4150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 80. | Goldberger JJ, Buxton AE. Personalized medicine vs guideline-based medicine. JAMA. 2013;309:2559-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 82. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 83. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5305] [Article Influence: 182.9] [Reference Citation Analysis (0)] |
| 84. | Chan SC. Liver transplantation for hepatocellular carcinoma. Liver Cancer. 2013;2:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 313] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 86. | Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, Takada Y, Uemoto S. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 87. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1570] [Article Influence: 92.4] [Reference Citation Analysis (1)] |
| 88. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 663] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 89. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1270] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 90. | Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 91. | Freeman RB, Mithoefer A, Ruthazer R, Nguyen K, Schore A, Harper A, Edwards E. Optimizing staging for hepatocellular carcinoma before liver transplantation: A retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 92. | Fujiki M, Aucejo F, Choi M, Kim R. Neo-adjuvant therapy for hepatocellular carcinoma before liver transplantation: where do we stand? World J Gastroenterol. 2014;20:5308-5319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 93. | Li HY, Wei YG, Yan LN, Li B. Salvage liver transplantation in the treatment of hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2012;18:2415-2422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Mergental H, Adam R, Ericzon BG, Kalicinski P, Mühlbacher F, Höckerstedt K, Klempnauer JL, Friman S, Broelsch CE, Mantion G. Liver transplantation for unresectable hepatocellular carcinoma in normal livers. J Hepatol. 2012;57:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 96. | Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2014;20:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 97. | Bralet MP, Régimbeau JM, Pineau P, Dubois S, Loas G, Degos F, Valla D, Belghiti J, Degott C, Terris B. Hepatocellular carcinoma occurring in nonfibrotic liver: epidemiologic and histopathologic analysis of 80 French cases. Hepatology. 2000;32:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 98. | Lubrano J, Huet E, Tsilividis B, François A, Goria O, Riachi G, Scotté M. Long-term outcome of liver resection for hepatocellular carcinoma in noncirrhotic nonfibrotic liver with no viral hepatitis or alcohol abuse. World J Surg. 2008;32:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Lang H, Sotiropoulos GC, Brokalaki EI, Schmitz KJ, Bertona C, Meyer G, Frilling A, Paul A, Malagó M, Broelsch CE. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg. 2007;205:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 100. | Smoot RL, Nagorney DM, Chandan VS, Que FG, Schleck CD, Harmsen WS, Kendrick ML. Resection of hepatocellular carcinoma in patients without cirrhosis. Br J Surg. 2011;98:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Faber W, Sharafi S, Stockmann M, Denecke T, Sinn B, Puhl G, Bahra M, Malinowski MB, Neuhaus P, Seehofer D. Long-term results of liver resection for hepatocellular carcinoma in noncirrhotic liver. Surgery. 2013;153:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 102. | Cauchy F, Fuks D, Belghiti J. HCC: current surgical treatment concepts. Langenbecks Arch Surg. 2012;397:681-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 103. | Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol. 2010;44:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 104. | Tan CK, Law NM, Ng HS, Machin D. Simple clinical prognostic model for hepatocellular carcinoma in developing countries and its validation. J Clin Oncol. 2003;21:2294-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 467] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 106. | Moser MA, Kneteman NM, Minuk GY. Research toward safer resection of the cirrhotic liver. HPB Surg. 2000;11:285-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Abdalla EK, Denys A, Hasegawa K, Leung TW, Makuuchi M, Murthy R, Ribero D, Zorzi D, Vauthey JN, Torzilli G. Treatment of large and advanced hepatocellular carcinoma. Ann Surg Oncol. 2008;15:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Ruzzenente A, Capra F, Pachera S, Iacono C, Piccirillo G, Lunardi M, Pistoso S, Valdegamberi A, D’Onofrio M, Guglielmi A. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg. 2009;13:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, Ikai I, Yamaoka Y, Curley SA, Nagorney DM. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 110. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 582] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 111. | Kuroda S, Tashiro H, Kobayashi T, Oshita A, Amano H, Ohdan H. Selection criteria for hepatectomy in patients with hepatocellular carcinoma classified as Child-Pugh class B. World J Surg. 2011;35:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Galun DA, Bulajic P, Zuvela M, Basaric D, Ille T, Milicevic MN. Is there any benefit from expanding the criteria for the resection of hepatocellular carcinoma in cirrhotic liver? Experience from a developing country. World J Surg. 2012;36:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 113. | Ho MC, Huang GT, Tsang YM, Lee PH, Chen DS, Sheu JC, Chen CH. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol. 2009;16:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 114. | Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10:S39-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 115. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 116. | van der Bilt JD, Livestro DP, Borren A, van Hillegersberg R, Borel Rinkes IH. European survey on the application of vascular clamping in liver surgery. Dig Surg. 2007;24:423-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 117. | van Gulik TM, de Graaf W, Dinant S, Busch OR, Gouma DJ. Vascular occlusion techniques during liver resection. Dig Surg. 2007;24:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 118. | Sugiyama Y, Ishizaki Y, Imamura H, Sugo H, Yoshimoto J, Kawasaki S. Effects of intermittent Pringle’s manoeuvre on cirrhotic compared with normal liver. Br J Surg. 2010;97:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 119. | Fu SY, Lau WY, Li GG, Tang QH, Li AJ, Pan ZY, Huang G, Yin L, Wu MC, Lai EC. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg. 2011;201:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Breitenstein S, Apestegui C, Petrowsky H, Clavien PA. “State of the art” in liver resection and living donor liver transplantation: a worldwide survey of 100 liver centers. World J Surg. 2009;33:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, Hawkins IF, Vauthey JN. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686-691; discussion 691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 122. | Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 404] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 123. | Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, Fürst G, Topp SA. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 124. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 932] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 125. | Vennarecci G, Laurenzi A, Levi Sandri GB, Busi Rizzi E, Cristofaro M, Montalbano M, Piselli P, Andreoli A, D’Offizi G, Ettorre GM. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol. 2014;40:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 126. | Vennarecci G, Laurenzi A, Santoro R, Colasanti M, Lepiane P, Ettorre GM. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg. 2014;38:1498-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 127. | Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 128. | Mise Y, Sakamoto Y, Ishizawa T, Kaneko J, Aoki T, Hasegawa K, Sugawara Y, Kokudo N. A worldwide survey of the current daily practice in liver surgery. Liver Cancer. 2013;2:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 129. | Arii S, Tanaka S, Mitsunori Y, Nakamura N, Kudo A, Noguchi N, Irie T. Surgical strategies for hepatocellular carcinoma with special reference to anatomical hepatic resection and intraoperative contrast-enhanced ultrasonography. Oncology. 2010;78 Suppl 1:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 130. | Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 131. | Shindoh J, Mise Y, Satou S, Sugawara Y, Kokudo N. The intersegmental plane of the liver is not always flat--tricks for anatomical liver resection. Ann Surg. 2010;251:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 132. | Ahn KS, Kang KJ, Park TJ, Kim YH, Lim TJ, Kwon JH. Benefit of systematic segmentectomy of the hepatocellular carcinoma: revisiting the dye injection method for various portal vein branches. Ann Surg. 2013;258:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 133. | Zhou YM, Shao WY, Zhao YF, Xu DH, Li B. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci. 2011;56:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 134. | Fancellu A, Rosman AS, Sanna V, Nigri GR, Zorcolo L, Pisano M, Melis M. Meta-analysis of trials comparing minimally-invasive and open liver resections for hepatocellular carcinoma. J Surg Res. 2011;171:e33-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 135. | Li N, Wu YR, Wu B, Lu MQ. Surgical and oncologic outcomes following laparoscopic versus open liver resection for hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2012;42:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 136. | Yin Z, Fan X, Ye H, Yin D, Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1203-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 137. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 138. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 634] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 139. | Lencioni R, Crocetti L. A critical appraisal of the literature on local ablative therapies for hepatocellular carcinoma. Clin Liver Dis. 2005;9:301-314, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 140. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 674] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 141. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 142. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 431] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 143. | Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, Wong J. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 144. | Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 145. | Lencioni R, Crocetti L. Image-guided thermal ablation of hepatocellular carcinoma. Crit Rev Oncol Hematol. 2008;66:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 146. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 529] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 147. | Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 294] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 148. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 149. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 150. | Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 151. | Liu Y, Zheng Y, Li S, Li B, Zhang Y, Yuan Y. Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol. 2013;68:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 152. | Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One. 2013;8:e76119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 153. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 154. | Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;CD004787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 155. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 156. | Dharancy S, Boitard J, Decaens T, Sergent G, Boleslawski E, Duvoux C, Vanlemmens C, Meyer C, Gugenheim J, Durand F. Comparison of two techniques of transarterial chemoembolization before liver transplantation for hepatocellular carcinoma: a case-control study. Liver Transpl. 2007;13:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 157. | Liu Z, Gao F, Yang G, Singh S, Lu M, Zhang T, Zhong Z, Zhang F, Tang R. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: an up-to-date meta-analysis. Tumour Biol. 2014;35:7407-7413. [PubMed] |
| 158. | Yang M, Yuan JQ, Bai M, Han GH. Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Mol Biol Rep. 2014;41:6575-6582. [PubMed] |
| 159. | Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e100305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 160. | Leng JJ, Xu YZ, Dong JH. Efficacy of transarterial chemoembolization for hepatocellular carcinoma with portal vein thrombosis: a meta-analysis. ANZ J Surg. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 161. | Kim DY, Ryu HJ, Choi JY, Park JY, Lee DY, Kim BK, Kim SU, Ahn SH, Chon CY, Han KH. Radiological response predicts survival following transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 162. | Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 163. | Cappelli A, Pettinato C, Golfieri R. Transarterial radioembolization using yttrium-90 microspheres in the treatment of hepatocellular carcinoma: a review on clinical utility and developments. J Hepatocellular Carcinoma. 2014;1:163-182. |
| 164. | Sangro B, Bilbao JI, Boan J, Martinez-Cuesta A, Benito A, Rodriguez J, Panizo A, Gil B, Inarrairaegui M, Herrero I. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 165. | Bilbao JI, de Martino A, de Luis E, Díaz-Dorronsoro L, Alonso-Burgos A, Martínez de la Cuesta A, Sangro B, García de Jalón JA. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: histological findings. Cardiovasc Intervent Radiol. 2009;32:727-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 166. | Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A, Nemcek AA, Gates VL. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 167. | Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 168. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 779] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 169. | Iñarrairaegui M, Thurston KG, Bilbao JI, D’Avola D, Rodriguez M, Arbizu J, Martinez-Cuesta A, Sangro B. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2010;21:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 170. | Lau WY, Sangro B, Chen PJ, Cheng SQ, Chow P, Lee RC, Leung T, Han KH, Poon RT. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology. 2013;84:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 171. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 172. | Lance C, McLennan G, Obuchowski N, Cheah G, Levitin A, Sands M, Spain J, Srinivas S, Shrikanthan S, Aucejo FN. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1697-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 173. | Golfieri R, Bilbao JI, Carpanese L, Cianni R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Cappelli A, Rodriguez M. Comparison of the survival and tolerability of radioembolization in elderly vs. younger patients with unresectable hepatocellular carcinoma. J Hepatol. 2013;59:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 174. | Ettorre GM, Santoro R, Puoti C, Sciuto R, Carpanese L, Antonini M, Antonucci G, Maini CL, Miglioresi L, Vennarecci G. Short-term follow-up of radioembolization with yttrium-90 microspheres before liver transplantation: new perspectives in advanced hepatocellular carcinoma. Transplantation. 2010;90:930-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 175. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10258] [Article Influence: 603.4] [Reference Citation Analysis (2)] |
| 176. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4648] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 177. | Smalley KS, Xiao M, Villanueva J, Nguyen TK, Flaherty KT, Letrero R, Van Belle P, Elder DE, Wang Y, Nathanson KL. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene. 2009;28:85-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 178. | Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1149] [Cited by in RCA: 1108] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 179. | Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 379] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 180. | Zhang Y, Xue D, Wang X, Lu M, Gao B, Qiao X. Screening of kinase inhibitors targeting BRAF for regulating autophagy based on kinase pathways. Mol Med Rep. 2014;9:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 181. | Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer. 2012;1:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 182. | Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, Shin YM, Kim KM, Lim YS, Lee HC. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 183. | Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 184. | Amar S, Wu KJ, Tan WW. Sorafenib-induced pancreatitis. Mayo Clin Proc. 2007;82:521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 185. | Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 186. | Product Information. Nexavar (sorafenib). West Haven, CT: Bayer Pharmaceutical Inc. Available from: http://www.univgraph.com/bayer/inserts/NexavarNAV_PI.pdf. |
| 187. | Alexandrescu DT, McClure R, Farzanmehr H, Dasanu CA. Secondary erythrocytosis produced by the tyrosine kinase inhibitors sunitinib and sorafenib. J Clin Oncol. 2008;26:4047-4048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 188. | Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, Ladrón de Guevara L, Papandreou C. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 189. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RTP, Han KH, Tak WY. STORM: A phase III randomized, double-blind, placebo-controlled trial of adjuvant sorafenib after resection or ablation to prevent recurrence of hepatocellular carcinoma (HCC). J Clin Oncol. 2014;32:5s. |
| 190. | Tabrizian P, Roayaie S, Schwartz ME. Current management of hepatocellular carcinoma. World J Gastroenterol. 2014;20:10223-10237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |