Published online Aug 8, 2015. doi: 10.4254/wjh.v7.i16.2041
Peer-review started: May 4, 2015
First decision: June 25, 2015
Revised: July 8, 2015
Accepted: July 23, 2015
Article in press: July 27, 2015
Published online: August 8, 2015
Processing time: 102 Days and 14.5 Hours
AIM: To review the effectiveness of exercise as a therapy for nonalcoholic fatty liver disease (NAFLD) and potential benefits in treating insulin resistance and atherosclerosis.
METHODS: Medline (EBSCOhost) and PubMed were searched for English-language randomized controlled trials and prospective cohort studies in human adults aged ≥ 18 which investigated the various effects of exercise alone, a combination of exercise and diet, or exercise and diet coupled with behavioral modification on NAFLD from 2010 to Feburary 2015.
RESULTS: Eighteen of 2298 available studies were chosen for critical review, which included 6925 patients. Nine (50%) studies were randomized controlled trials. Five (27.8%) studies utilized biopsy to examine the effects of physical activity on hepatic histology. The most commonly employed imaging modality to determine change in hepatic steatosis was hydrogen-magnetic resonance spectroscopy. Only two studies examined the effects of low impact physical activity for patients with significant mobility limitations and one compared the efficacy of aerobic and resistance exercise. No studies examined the exact duration of exercise required for hepatic and metabolic improvement in NAFLD.
CONCLUSION: While exercise improved hepatic steatosis and underlying metabolic abnormalities in NAFLD, more studies are needed to define the most beneficial form and duration of exercise treatment.
Core tip: Lifestyle modification through increased physical activity is beneficial in patients with nonalcoholic fatty liver disease (NAFLD). Although weight loss has been shown to produce improvement in biochemical and histological markers of NAFLD, exercise might improve hepatic steatosis and steatohepatitis even in the absence of major weight loss. Cardiovascular and resistance training both seem to benefit patients with NAFLD; further study is needed to determine if one is more effective than the other. A reduction in sedentary time in the absence of increased intense physical activity might also improve NAFLD, although more research is required.
- Citation: Whitsett M, VanWagner LB. Physical activity as a treatment of non-alcoholic fatty liver disease: A systematic review. World J Hepatol 2015; 7(16): 2041-2052
- URL: https://www.wjgnet.com/1948-5182/full/v7/i16/2041.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i16.2041
Non-alcoholic fatty liver disease (NAFLD) is a burdensome and increasingly prevalent disease throughout the world. It is the most common cause of chronic liver disease among children and adolescents and represents the leading cause of chronic liver disease worldwide[1]. The staggering prevalence of NAFLD, by some estimates affecting more than 30% of the Western population, parallels the increasingly sedentary lifestyle and continued rise of the obesity epidemic[2]. NAFLD is commonly referred to as the liver manifestation of the metabolic syndrome, and risk factors for its development include diabetes, obesity, and hyperlipidemia[3]. The prevalence of NAFLD approaches 90% in patients with hyperlipidemia, 70% in patients with type 2 diabetes, and greater than 91% in patients who undergo bariatric surgery[4-6].
NAFLD encompasses a spectrum of disease ranging from isolated hepatic steatosis to steatosis with inflammation and hepatocyte injury [non-alcoholic steatohepatitis (NASH)], which is an increasingly common cause of cirrhosis and hepatocellular carcinoma and is on trajectory to become the most common indication for liver transplantation in the United States[7]. Patients with concurrent diabetes mellitus are at a higher risk for the development of NASH, particularly as insulin sensitivity worsens. Diabetics with NASH experience higher rates of microvascular complications, such as chronic kidney disease and retinopathy, as well as higher rates of all-cause mortality when compared to non-NASH diabetics[3,8]. In addition, NAFLD is associated with prevalent coronary artery disease and myocardial dysfunction[9]. A diagnosis of NAFLD is an independent risk factor for the development of cardiovascular disease, which represents the leading cause of morbidity and mortality in this patient population[9,10]. Early recognition and treatment of NAFLD is crucial in the prevention of associated cardiometabolic and liver-related mortality.
While numerous pharmacologic agents can be used to treat the metabolic derangements that often coexist in NAFLD, pharmacologic treatments for NAFLD itself are lacking. The first line treatment of NAFLD is lifestyle intervention, including diet and exercise[11,12]. Exercise may aid in the reduction of hepatic steatosis, prevent progression to cirrhosis, and may improve both insulin sensitivity and cardiovascular health, factors which contribute to the leading cause of mortality in this patient population[12]. Despite the well-established benefits of exercise, there is a lack of robust data to support the efficacy of exercise as treatment in NAFLD[13]. Data gleaned from cross sectional studies correlate inactivity and sedentary lifestyles with the development of NAFLD[14,15]. Physical inactivity leads to reduced insulin sensitivity, an increase in visceral and peripheral fat deposition, and an increase in free fatty acid uptake by the liver[16]. Exercise as a treatment for NAFLD targets many aspects of the disease: the metabolic syndrome, insulin resistance, hepatic steatosis, and cardiovascular disease. Exercise also thwarts the proposed two step development of steatohepatitis, which occurs as a result of deranged fatty acid and lipid metabolism, leading to increased deposition and impaired export of hepatic lipids along with de novo lipogenesis, followed by an increase in inflammatory cytokines and infiltrate within the liver[17,18].
Current guidelines do not address specific recommendations for exercise therapy among persons with NAFLD, such as which form of exercise, level of intensity, or duration of treatment provides the most benefit for NAFLD reduction. Well-designed studies in diabetic populations, a population that shares a similar physiology with NAFLD, suggest that combination exercise with both aerobic and resistance exercise achieves the greatest improvement in metabolic parameters including glucose control and abdominal adiposity, and it reduces the risk of developing cardiovascular disease and the microvascular complications of diabetes[19,20]. The aim of the current study is to conduct a systematic review of the available published literature to assess the efficacy of exercise as a treatment for NAFLD and its effect on the cardiometabolic comorbidities of NAFLD, including insulin resistance, dyslipidemia and amount of visceral adiposity. Various forms of physical activity treatment will be reviewed, including exercise programs with and without controlled diets and exercise of varying intensity, duration, and form.
This systematic review adheres to the relevant criteria from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. The methods used, including identification, screening, eligibility, and inclusion, were agreed by the authors (Whitsett M and VanWagner LB) in advance. An electronic search of the English language medical literature was conducted using Medline (EBSCOhost) and PubMed to identify published articles on the role of physical activity as a treatment for NAFLD in adults aged ≥ 18 years of age. This search strategy used a combination of the following prespecified MeSH headings and keywords alone or in combination: “NAFLD”, “nonalcoholic fatty liver disease”, “fatty liver”, “hepatic steatosis”, “NASH”, “nonalcoholic steatohepatitis”, “non-alcoholic steatosis”, “exercise”, “resistance training”, “aerobic training”, “aerobic exercise”, “circuit training”, “walk test”, “endurance training”, “strength training”, “weight training”. Boolean operators (“and”, “or”) were also used in succession to narrow or widen the search. The search was restricted to English language and human studies.
Studies examining the association between physical activity in adult patients with NAFLD were included. Randomized controlled trials, prospective cohort trials, and well-constructed retrospective studies were included.
Non-English language studies and animal studies were excluded. Studies which examined adolescents or children (age < 18) were excluded.
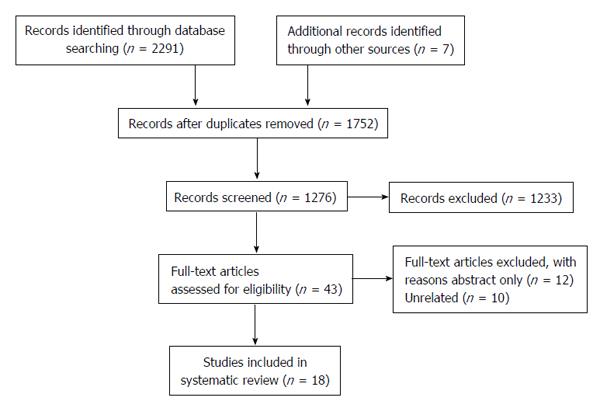
A total of 2298 studies were initially identified through a comprehensive database search. An additional 7 studies were identified from a hand review of references. After duplicate removal and screening for studies published from 2010 until February 2015, 1276 studies were identified. Fifty-three relevant studies were screened through review of article title and abstract, and eighteen studies were included in this review (Figure 1). Nine (50%) of these studies were randomized controlled trials. A total of 6925 patients were included in these studies. Five (27.8%) studies utilized biopsy to examine the effects on hepatic histology, and the most commonly employed imaging modality to determine change in hepatic steatosis was hydrogen-magnetic resonance spectroscopy (H-MRS). Two studies examined the effects of low impact physical activity for patients with significant mobility limitations. One study compared the efficacy of aerobic and resistance exercise in NAFLD patients. Study characteristics are summarized in Table 1. Main study findings will be discussed below and are summarized in Table 2.
| Ref. | n | NAFLD assessment | Design | Parameter studied | Type of activity intervention | Nutrition intervention | Exercise | |||
| Frequency | Intensity | Duration | Intervention duration | |||||||
| Hallsworth et al[26] | 37 | H-MRS | P | Sedentary and physical activity time | Activity monitor | No | NA | NA | NA | 7 d |
| Gerber et al[14] | 1263 | Fatty liver index > 60 | C | Level of physical activity | Activity monitor | No | NA | NA | NA | 7 d |
| Oh et al[33] | 18 | US, H-MRS | P | Hepatic steatosis | Acceleration | No | 2 × wk | NA | 20 min | 12 wk |
| Kawaguchi et al[32] | 35 | US | P | Hepatic steatosis | Hybrid | Yes | 2 × wk | NA | 20 min | 12 wk |
| Kistler et al[34] | 813 | Liver biopsy | R | Physical activity, NAS | None (self-report) | No | NA | Inactive, moderate, vigorous | NA | NA |
| Haus et al[36] | 17 | H-MRS | P | IR, intrahepatic TG content | Aerobic | No | Consecutive days | 80%-85% max heart rate | 50-60 min | 7 d |
| Promrat et al[38] | 31 | Liver biopsy | RCT | NAS | Aerobic | Yes | Weekly | Moderate | 200 min | 48 wk |
| Pugh et al[39] | 13 | US, liver enzymes | RCT | Cutaneous microvascular function | Aerobic | No | 3 × wk | Moderate | 30-45 min | 16 wk |
| Pugh et al[40] | 34 | US, liver enzymes | RCT | Microvascular function | Aerobic | No | 3 × wk | Moderate | 30-45 min | 16 wk |
| Sullivan et al[27] | 18 | H-MRS | RCT | Intrahepatic TG content, lipid kinetics | Aerobic | No | 5 × wk | Moderate | 30-60 min | 16 wk |
| Jin et al[23] | 120 | Liver biopsy | R | Steatosis by histology | Aerobic | Yes | 3 × wk | NA | 20 min | No set length |
| Oh et al[28] | 52 | US | P | Hepatic steatosis | Aerobic | Yes | 3 × wk | Max HR > 40% | 90 min | 3 mo |
| Sun et al[24] | 1087 | US, liver enzymes | RCT | Metabolic parameters | Aerobic | Yes | NA | NA | NA | 12 mo |
| Zelber-Sagi et al[30] | 82 | US | RCT | Hepatic steatosis | Resistance | No | 3 × wk | NA | 40 min | 3 mo |
| Hallsworth et al[29] | 21 | H-MRS | RCT | Intrahepatic lipid content | Resistance | No | 3 × wk | NA | 45-50 min | 8 wk |
| Bacchi et al[43] | 31 | MRI | RCT | Hepatic steatosis | Resistance and aerobic | No | 3 × wk | Moderate | 60 min | 4 mo |
| Eckard et al[21] | 41 | Liver biopsy | P | Histology | Resistance and aerobic | Yes | 4-7 × wk | Moderate | 20-60 min | 6 mo |
| Oh et al[35] | 169 | US | RCT | Hepatic steatosis | Aerobic | Yes | Weekly | Vigorous | 150->250 min | 12 wk |
| Ref. | Population | Type of activity | Weight loss | Insulin resistance | Inflammation and | Liver | Liver fat | Liver | Conclusions |
| intervention | (% Δ) | and lipids | oxidative stress | enzymes | by imaging | histology | |||
| Hallsworth et al[26] | NAFLD | Activity monitor | NA | NA | NA | NA | NA | NA | NAFLD = more sedentary time, less energy expenditure, and greater prevalence of DM than healthy controls |
| Gerber et al[14] | NAFLD, NAFLD + DM | Activity monitor | NA | NA | NA | NA | NA | NA | NAFLD = less PA time than non-NAFLD NAFLD + DM = lowest quartile of average PA as well as moderate-vigorous PA |
| Oh et al[33] | NAFLD | Acceleration | -1.9% | NA | ↓ TNF-α, IL-6, leptin, IMCL ↑ adiponectin | Improved | Improved (US) | NA | Acceleration training results in significant improvement in IR, inflammation, liver enzymes, steatosis and quality of life |
| Kawaguchi et al[32] | NAFLD | Hybrid | -0.9% | ↓ Insulin HOMA-IR | ↓ IL-6 | Improved | Improved (US) | NA | Hybrid training results in significant improvement in IR, inflammation, liver enzymes and steatosis |
| Kistler et al[34] | NASH, NAFLD | None (self-report) | NA | ↓ Insulin and glucose (vigorous PA vs inactive) | No effect | No effect | NA | Vigorous PA = ↓ odds of NASH and fibrosis | Vigorous but not moderate or total exercise is associated with the severity of NAFLD |
| Haus et al[36] | NAFLD | Aerobic | 0 | ↓ Glucose HOMA-IR | ↑ Lipid PUI, adiponectin | NA | NA | Improved steatosis | Short-term aerobic exercise favorably alters hepatic lipid composition by increasing polyunsaturated lipids |
| Promrat et al[38] | NASH | Aerobic | -9.3% | ↓ Glucose, insulin, HbA1C HOMA-IR (NS) | NA | Improved | NA | Improved NAS. No change in fibrosis | > 7% weight loss resulted in improvement in overall NAS, ballooning, steatosis, inflammation |
| Pugh et al[39] | NAFLD | Aerobic | 0 | No effect | No effect | Improved | No effect | NA | Aerobic exercise improves NO-mediated vasodilation in NAFLD |
| Pugh et al[40] | NAFLD | Aerobic | 0 | ↓ Glucose | No effect | No effect | No effect | NA | Aerobic exercise improves flow mediated dilation in NAFLD |
| Sullivan et al[27] | NAFLD | Aerobic | 0 | No effect | No effect | Improved | Improved (H-MRS) | NA | Aerobic exercise without weight loss results in significant reduction in intrahepatic triglyceride content |
| Jin et al[23] | NAFLD | Aerobic | -3.9% | ↓ Total cholesterol | NA | Improved | NA | Improved steatosis | Aerobic exercise results in decreased steatosis among living donors even in the absence of significant weight loss |
| Oh et al[28] | NAFLD | Aerobic | -13.3% | ↓ HbA1C HOMA-IR, LDL, TG ↑ insulin, HDL | ↓ TNF-α, IL-6, leptin, hsCRP, ferritin, TBARS ↑ adiponectin | Improved | Improved (US and Fibroscan) | NA | Diet with exercise exceeds diet alone in reducing steatosis, inflammation, insulin resistance |
| Sun et al[24] | NAFLD | Aerobic | -11.6% | ↓ HOMA-IR, total cholesterol | No effect | Improved | NA | NA | Aerobic exercise results in decrease in ALT, insulin resistance, and the metabolic syndrome |
| Zelber-Sagi et al[30] | NAFLD | Resistance | -0.75% | ↓ Total cholesterol | ↓ Ferritin | No effect | Improved (US) | NA | Resistance exercise results in reduction in steatosis, abdominal adiposity, inflammation, cholesterol |
| Hallsworth et al[29] | NAFLD | Resistance | 0 | ↓ HOMA-IR (NS) | ↑ Fat oxidation | No effect | Improved (H-MRS) | NA | Resistance exercise results in a 13% relative reduction in intrahepatic lipids |
| Bacchi et al[43] | NAFLD + DM | Resistance vs aerobic | No | ↓ HDL, TG, HbA1c ↑ clamp-measured insulin sensitivity | No effect | No effect | Improved (MRI) | NA | Both resistance and aerobic exercise result in improved steatosis, abdominal, and visceral fat content |
| Eckard et al[21] | NAFLD, NASH (88%) | Resistance and aerobic | -1.3%1 | No effect | No effect | Improved | NA | Improved NAS in all groups | Lifestyle modification, even without weight loss, improves NAS |
| Oh et al[35] | NAFLD | Aerobic | 10.4%2 | ↓ HOMA-IR, LDL, TG ↑ HDL | ↓ TNF-α, IL-6, leptin, hsCRP, ferritin, TBARS ↑ adiponectin | Improved | Improved (US, fibroscan) | NA | Moderate to vigorous PA (> 250 min weekly) significantly reduces markers of IR, oxidative stress and fatty acid metabolism independent of weight reduction |
Eckard et al[21] examined how variations in diet with moderate intensity exercise regimens impact the NAFLD activity score (NAS), which evaluates the degree of hepatocellular ballooning, steatosis, and inflammatory infiltrate on liver biopsy[22]. He enrolled 56 participants in four distinct groups: (1) standard control (n = 14); (2) low fat diet with moderate exercise (n = 14); (3) moderate fat/low processed carbohydrate diet with moderate exercise (n = 13); and (4) moderate exercise only (n = 15). For six months, participants in the exercise arms engaged in an 18-step exercise program that combined aerobic and resistance exercises for 20-60 min, 4-7 d per week. Participants assigned to the low fat and moderate fat groups received nutritional instruction, attended special nutrition classes, and received personalized diet plans, designed with a goal of achieving one pound of weight loss per week based on caloric intake and energy expenditure. All participants received instructions on the completion of a 3-d food log. Participants in the standard care group also attended one specialized nutrition class. Participants in the low fat and moderate fat groups received nutritional follow-up counseling and specific dietary education. Biopsies were performed before and after the intervention to determine the change in NAS. All groups experienced a significant decrease in NAS from baseline, yet there was no statistically significant difference between groups. Additionally, there was a significant decrease in aminotransferase levels. Not one group achieved a significant degree of weight loss. Of note, there was a high attrition rate in this particular study, with a loss of fifteen participants. Additionally, 50% of participants in both the low fat and moderate fat groups were non-adherent to their prescribed diet and caloric expenditure. Because of the complexity of the diet intervention as well as the poor adherence to the prescribed medium and low fat diets, it is difficult to ascertain what effect, if any, dietary changes had on NAFLD. However, this study suggests that exercise without weight loss can still achieve an improvement in the health of NAFLD patients.
Jin et al[23] performed a retrospective study to determine how aerobic exercise and lifestyle changes improved steatosis in 120 non-obese potential living liver donors found to have NAFLD on routine biopsy. Because graft survival after liver transplantation is heavily impacted by the degree of hepatic steatosis of the donor, it is imperative that donors maintain healthy lifestyles and engage in physical activity prior to donation. Patients originally biopsied and found to have moderate to severe steatosis (greater than 30%-60% hepatocytes on biopsy with fat granules) or mild steatosis (5%-30%) with graft-to-recipient weight ratio < 0.8 were encouraged to reduce total body weight by 5% through diet and aerobic exercise. No patients had fibrosis or NASH within the trial. The regimen consisted of aerobic exercises, thrice weekly for at least 20 min per session, and the diet recommended was 25 cal/kg × ideal body weight (kg) consisting of 50% carbohydrates, 20% protein, and 30% fat. Patients were re-biopsied according to the health status of the recipient or once they had achieved a loss of 5% or more of their total body weight. Eighty-five percent of donors (103 of 120 patients), including some who maintained the same weight or lost none, experienced an improvement in steatosis. In total, the group experienced a 21.3% reduction in total steatosis. Thus, not only can steatosis improve significantly when diet and exercise lead to weight loss, but patients who partake in aerobic exercise and diet without experiencing weight loss can still achieve improvement in steatosis.
Sun et al[24] launched an impressively large study in China by studying the effect of lifestyle intervention on various metabolic parameters in 1087 NAFLD patients over the course of one year. Patients were randomized to control or lifestyle; the lifestyle intervention group was given a special diet: 30% fat, 15% protein, and 35% carbohydrates. They were encouraged to walk, jog, stair climb, and do other exercises with a goal of 23 metabolic equivalent tasks (METs)•h/week (physical activity) + 4 METs•h/week (exercise). Those in the control group were encouraged to maintain their everyday habits and were provided education on the value of healthy eating and exercise in NAFLD. Patients in the lifestyle arm experienced a significant reduction in alanine aminotransferase (ALT), insulin resistance, and prevalence of the metabolic syndrome when compared to controls. While the study showed that the group undergoing lifestyle intervention experienced a significant improvement in their health, it is difficult to ascertain the true benefit of exercise with the lack of controlling for diet.
Activity levels in patients with NAFLD: Living a sedentary lifestyle can lead to the development of obesity and insulin resistance, factors which then can predispose to the metabolic syndrome. Sedentary time is defined as periods of both lengthy periods of inactivity and lack of movement, such as in television watching, sleeping, or sitting[25]. Previous studies gauging sedentary behavior in NAFLD patients relied on subjective data, surveys mostly, to reach their conclusions. Hallsworth et al[26] objectively measured the extent of physical inactivity and sedentary time in NAFLD patients. He provided wearable devices to all participants, which measured activity level and energy expenditure over the course of seven days. As expected, NAFLD patients reported having longer periods of sedentary time, less energy expenditure, and less walking time and active time than healthy controls. While the NAFLD patients had on average a higher magnetic resonance imaging (BMI) and weight, no comparison could be made regarding the degree of hepatic steatosis or insulin resistance between case and control due to the fact that only NAFLD patients received laboratory evaluation. Gerber et al[14] gleaned data from the National Health and Nutrition Examination Survey from 2003-2004 and 2005-2006 to examine 3 groups: NAFLD alone, NAFLD plus diabetes, and healthy controls. He assessed the difference in physical activity between the groups by the use of specially designed activity monitors. NAFLD patients were found to be less physically active, have increased sedentary time, and have a modest prevalence of diabetes. Neither study offered any exercise intervention per se, but it is important to note that in addition to encouraging formal exercise, minimizing sedentary time may be another approach to minimize the deleterious effects of NAFLD.
Aerobic activity and NAFLD: Sullivan et al[27] sought to examine the relationship between aerobic exercise and hepatic lipid levels, hypothesizing that moderate intensity aerobic exercise would not only reduce triglyceride content within the liver but that it would decrease the rate of secretion of very low density lipoprotein triglycerides (VLDL-TG) by the liver. Eighteen patients were divided into the exercise arm, which required exercising at 45%-55% peak oxygen consumption (VO2) rate for 16 wk with 30-60 min sessions held 5 times weekly. There was not a dietary component to either arm of the study, and control patients were told to continue with their daily activities. Patients underwent H-MRS pre and post intervention to evaluate the triglyceride content of their livers, and underwent a study with isotope tracer to determine if the VLDL secretion rates had improved. After the intervention, the concentration of intrahepatic triglyceride content in the exercise arm decreased significantly (> 10%), without a significant change in body composition or weight. The reduction of hepatic triglycerides also correlated with a reduction in serum ALT. Exercise had no significant effect on VLDL-TG or VLDL-apoB100 secretion rates or lipid plasma concentration. While the study evaluated a small number of patients, the results bolster the body of evidence that suggests that exercise with a moderate level of intensity has a modest impact on the degree of hepatic steatosis in NAFLD patients.
Oh et al[28] studied how a regimented diet in conjunction with aerobic exercise can improve hepatic steatosis and various anthropometric parameters, such as visceral and subcutaneous adiposity, when compared to diet alone. Fifty-two obese men with NAFLD either engaged in diet alone or diet plus aerobic exercise which consisted of either walking or jogging for 90 min thrice weekly at a maximum heart rate > 40%. The diet was 1680 kcal daily, and patients kept food journals, met with dietitians, and attended group education sessions. The diet and exercise group experienced a greater improvement in inflammatory serum markers as well as a greater reduction in hepatic steatosis, visceral and subcutaneous adiposity, and insulin resistance compared to patients who only dieted. Cardiorespiratory fitness, as measured by VO2max, improved in both arms but to a greater degree in the combined diet and exercise cohort. Also, there was a significant correlation between the volume of exercise (measured by the change in number of steps) and the degree of reduction of steatosis, suggesting that greater duration of exercise produces an appreciable difference in markers of hepatic function. Thus, diet coupled with exercise has an overall greater benefit than diet alone in improving body habitus and markers of inflammation and oxidative stress in NAFLD patients.
Resistance training and NAFLD: Resistance exercise may be more feasible for certain subgroups of NAFLD patients, particularly for those with poor cardiorespiratory fitness or those who are overweight and cannot, due to body habitus, tolerate or participate in aerobic fitness. Hallsworth et al[29] examined the effect of resistance training without weight loss on NAFLD patients with sedentary lifestyles, defined as less than 60 min of vigorous activity daily. The study did not include dietary intervention, and the diets of the participants were unknown. After eight weeks of a structured exercise program targeting various muscle groups and with progressive increase in the amount of resistance, researchers found that even in those patients who did not lose weight, the resistance exercise group had a significant reduction in hepatic steatosis as well as an improvement in glycemic control and lipid oxidation. In both the control and resistance groups, BMI remained relatively stable, and there was no significant change in ALT or lipids. Thus, in NAFLD, it is possible to achieve improvement in hepatic steatosis, insulin resistance, and lipid oxidation without losing weight. Therefore, patients with functional limitations or poor cardiorespiratory fitness who may struggle with the demands of aerobic exercise can still benefit from resistance training.
Zelber-Sagi et al[30] sought to investigate the effect of resistance training on NAFLD in reducing hepatic steatosis, as measured by ultrasound and the hepatorenal index, a ratio which compares the echogenicity of the liver and kidney to quantify the degree of hepatic steatosis. This form of exercise was compared to home stretching exercises[31]. Patients in the treatment arm completed a three-month program comprised of resistance exercises with the goal of progressively increasing the intensity of resistance. The workouts were self- monitored and specifically avoided aerobic exercise. The stretching arm was provided stretching exercises targeted to eight different muscle groups; stretching was performed three days per week. There were no dietary restrictions, and at the start and completion of the intervention patients provided information regarding their nutritional intake.
Zelber-Sagi et al[30] reported that there was a significant reduction for the resistance arm in the hepatorenal index (11% vs 3.5%), a significantly greater reduction in hepatic and abdominal adiposity as well as a decrease in serum ferritin and cholesterol compared to the stretching group. Thus, not only is resistance exercise an important adjunct to aerobic exercise in treating steatosis, but it seems to help mitigate aspects of the inflammatory environment, which are thought to contribute to the pathogenesis of cardiovascular disease and progressive steatohepatitis among NAFLD patients.
There is a subset of NAFLD patients in whom moderate aerobic or resistance exercise is exceptionally difficult (the morbidly obese, incapacitated or bedridden, elderly, or those with other mobility-limiting comorbidities). More rigorous forms of exercise may also be unsafe for patients to complete. Hybrid training consists of simultaneous voluntary muscular contraction and electrical stimulation of the opposing muscle group. Kawaguchi et al[32] examined the efficacy of hybrid training to improve the metabolic consequences of NAFLD. In this study, patients performed both knee flexion and extension exercises. Hybrid training does not require a patient to be standing, thus these exercises can be performed while in bed and could potentially be of benefit to patients with low mobility. All 35 patients enrolled received 12 wk of nutritional counseling. Patients in the control group were advised to consume less than 25% of total calories from fat and consume a diet consisting of 25-30 kcal/kg per ideal body weight. Those in the hybrid group had 24 total exercise sessions, twice weekly for 12 wk. There was a significant decrease in body weight, body fat, serum ALT, hepatic steatosis, and insulin resistance. There was no effect on serum lipids or basal metabolic rate. Low intensity exercise, such as hybrid training, offers promise to NAFLD patients who are debilitated by their illness or other comorbidities.
Another type of exercise that can be used in relatively immobile patients is acceleration training. Acceleration training is a new training method that provides a physical stimulation effect on skeletal muscles by increasing gravitational acceleration with vibration. Participants either hold particular poses or engage in dynamic movements to activate muscle fibers and increase muscular endurance and strength. Oh et al[33] studied the effect of acceleration training on obese patients with NAFLD who previously struggled with weight loss in another study. Eighteen obese NAFLD patients who had completed a 12-wk counseling program for lifestyle changes without experiencing an improvement in hepatic enzymes or steatosis were chosen for this study. The exercise program consisted of acceleration training and utilized whole body three dimensional vibration on a special platform. There was no specific diet for this study; however, patients did receive dietary education as well as keep a food log for three days. At the end of the 12 wk of training, there was a significant improvement in anthropometry and intramyocellular lipid content. Intrahepatic steatosis decreased by 8.7%. Also notable were the reported improvements in quality of life and mental health of the patients after the intervention, which factors positively into motivation and willingness to make lifestyle changes. While this is a relatively new form of exercise explored in NAFLD, it may be a promising alternative to traditional exercise in certain subpopulations of NAFLD.
For those patients who can exercise without limitations, questions remain over what frequency, intensity, and duration is sufficient to improve features of NAFLD. Kistler et al[34] performed a retrospective analysis of self-reported physical activity levels and sought to explore the association between the histopathology of NAFLD and the volume and intensity of the reported exercise regimen. The authors posited that individuals who met moderate to vigorous exercise recommendations would have less fibrosis on pathology and have a lower frequency of NASH. Researchers examined survey results and correlated these with liver biopsy pathology of 813 adults with NAFLD enrolled in two trials from the NASH Clinical Research Network. Patients reported the volume, type, and intensity level of exercise as measured by metabolic equivalent values. No dietary intervention occurred in either of the trials. A large proportion of these patients did not achieve adequate volume or intensity of exercise, with 54% of those polled reporting inactive lifestyles. Those who reported vigorous activity (26% of patients) had lower serum insulin, gamma-glutamyl transferase, and glucose levels. Additionally, patients fulfilling the minimum requirements for vigorous activity experienced a significant reduction in their adjusted odds of having NASH (OR = 0.65, 95%CI: 0.43-0.98, P = 0.04). Those exceeding minimum requirements had a significantly lower odds of having advanced fibrosis (OR = 0.56, 95%CI: 0.34-0.90, P = 0.02). Thus, the intensity of exercise had a greater role in improving NAFLD than the volume or duration of intervention.
Oh et al[35] examined the benefits of varying degrees of intensity in exercise programs and how the intensity level impacted the degree of hepatic steatosis. Patients were divided into three groups: exercise < 150 min/wk, 150-250 min/wk, > 250 min/wk. The patients exercised for 12 wk total and were instructed to remain on a strict diet of 1680 kcal daily. A uniaxial accelerometer was used on patients to measure energy expenditure. At the study’s completion, all groups experienced a significant reduction in weight and BMI. Patients exercising > 150 min weekly achieved a reduction in weight of 12.4% as well as an improvement in hepatic steatosis. Those who exercised for 250 min or longer per week experienced an improvement in hepatic steatosis, ferritin, and other inflammatory markers. Thus, moderate to vigorous exercise of 250 min weekly provided anti-oxidative and anti-inflammatory benefits to NAFLD patients.
To explore the optimal duration of exercise, Haus et al[36] investigated the role of short duration (< 7 d) exercise in affecting hepatic steatosis. Researchers hypothesized that even short periods of aerobic exercise could be beneficial in NAFLD by leading to a change in lipid composition of the liver, reducing pro-inflammatory substances, and improving insulin sensitivity[37]. Seventeen obese, non-diabetic NAFLD patients completed a 7-d course of aerobic exercise comprised of walking or jogging for 50-60 min daily at 80%-85% maximum heart rate. H-MRS scans were performed before and after the intervention. Researchers demonstrated that there was an increase in polyunsaturated lipid content in the liver post-exercise. Also, they observed an increase in serum adiponectin, an anti-inflammatory molecule that regulates lipid oxidation. The findings of this study are consistent with known benefits of exercise: improvement in insulin sensitivity and a reduction in the formation of damaging reactive oxygen species.
Most of the studies reviewed in this paper contain a wide range of patients within the spectrum of fatty liver disease, but the most at-risk group for poor outcomes are those with NASH. Promrat et al[38] recruited patients with biopsy-proven NASH to determine if achieving a loss of 7%-10% of body weight through behavioral modification, exercise, and diet would achieve histologic improvement of NASH[39]. Researchers randomized NASH patients to either exercise or a control group for 48 wk. The control group received education on lifestyle modification, diet, and exercise. The lifestyle intervention arm combined behavioral strategies, individualized diets, and moderate intensity exercise. The caloric allotment in the diets was based upon a patient’s starting weight, and the goal was to achieve a 0.5-1 pound weight loss weekly. Patients chose their own exercise and were encouraged to walk 10000 steps daily and achieve 200 min weekly of exercise. On biopsy, the lifestyle intervention group had a significantly larger improvement in NAS. Both groups experienced improvement in ballooning score, and neither group experienced a change in fibrosis score. Those who lost greater than or equal to 7% total body weight had a significant improvement in NAS, hepatic steatosis, ballooning, and inflammation. The lifestyle intervention group experienced a significantly larger mean weight change (-8.7 kg vs -0.5 kg) and percentage weight reduction than controls at 24 and 48 wk. The fact that a number of patients (67% vs 20%, P = 0.02), enrolled in the intervention arm experienced a complete resolution of NASH on biopsy is important, as these patients are at high risk of adverse clinical outcomes if not treated promptly. However, exercise was again combined withdiet and behavioral modification so the incremental effect of moderate intensity exercise cannot be elucidated from this trial.
Because the leading cause of morbidity and mortality among patients with NAFLD is due to cardiovascular disease, researchers are interested in understanding how exercise mitigates the factors present in NAFLD that predispose to atherosclerosis. An impairment in the body’s ability to properly regulate microvascular circulation occurs early in the atherogenic process. Pugh et al[40] examined how exercise impacts the microvascular function of cutaneous vessels through measuring the release of nitric oxide (NO) in NAFLD patients. NO is an important mediator in vasodilation during exercise, and it is known that a deficiency of NO contributes to inflammation and lipid deposition within vessel walls. To assess patients’ cutaneous blood flow, researchers placed microdialysis probes into cutaneous vessels. Doppler probe signals generated then allowed the researchers to calculate cutaneous vascular conductance. Fourteen patients with NAFLD were assigned to either fully-supervised exercise training or conventional group which received information on lifestyle modification and encouragement to exercise. The exercise arm participated in moderate intensity exercise, three times weekly at 30% of heart rate reserve. The patients eventually escalated to five sessions per week of 45 min duration. Researchers found that as the patients’ skin heats from exercise, they experienced increasing amounts of NO release and vasodilation. This improved with time as patients progressed in their exercise routines. These findings suggest that exercise of any sort may help to reduce the risk of cardiovascular disease by improving microvascular function throughout the body.
Pugh et al[40] then sought to establish a relationship between NAFLD and endothelial function[41]. Additionally, researchers also wanted to determine if the amount of visceral and hepatic fat would correlate with the degree of endothelial impairment. Endothelial dysfunction is a prerequisite for the development of atherosclerosis and subsequent cardiovascular disease[42]. Endothelial function was measured by flowmediated dilation (FMD) of the brachial artery in 21 patients with NAFLD. Thirteen patients participated in moderate intensity exercise, consisting of supervised and individualized programs. At initiation, patients exercised for 30 min weekly three times per week at 30% of heart rate reserve and then progressed to 45 min sessions five times weekly. There was no specific diet that patients followed. The control group received teaching sessions on exercise and healthy eating, and these patients were permitted to exercise if they wanted. There was a significant improvement in FMD among the exercise group (3.6%, 95%CI: 1.6-5.7, P = 0.002). Visceral and hepatic fat did not necessarily influence the degree of FMD. Thus, moderate intensity exercise can improve the endothelial function of conduit arteries regardless of improvement in hepatic or visceral adiposity.
Exercise regimens have also been rigorously evaluated in diabetic patients with NAFLD. In the literature, there is a similar dilemma regarding exercise and diabetic patients, as it is unclear which form of exercise provides the most benefit for glycemic control and improving cardiovascular fitness[43]. Bacchi et al[43] examined how a combination of aerobic and resistance exercise impacts insulin sensitivity and adiposity of the liver, viscera, and midsection in patients with both diabetes and NAFLD. This randomized control trial was derived from a trial which that compared the metabolic effects of supervised resistance vs aerobic exercise for type 2 diabetics with sedentary lifestyles. The aerobic arm participated in 60-min sessions on various cardio machines, exercising at a goal of 60%-65% of their heart rate reserve. The resistance arm participated in weight lifting exercises with 3 series of 10 repetitions. Both groups exercised thrice weekly for four months and maintained their old diets. There was a significant reduction in hepatic adiposity in both groups, and nearly 25% of patients in both groups no longer had hepatic steatosis at the end of the trial. Insulin sensitivity, glycosylated hemoglobin, and high density lipoprotein serum levels were reduced similarly in both groups, as was abdominal and visceral adiposity. Thus, among diabetics with NAFLD, resistance and aerobic exercise both result in a reduction in hepatic steatosis and one form of exercise is not superior to another.
The benefit of exercise in NAFLD patients is undeniable and extends well beyond the liver by improving metabolic derangements such as insulin resistance and atherosclerotic disease and facilitating the development of cardiorespiratory fitness, among others. Since Keating’s comprehensive systematic review in 2012 on the effects of exercise on NAFLD, more data has surfaced in support of exercise and lifestyle behavior modification, and recent studies have attempted to explore and define the potential benefits of exercise on both the liver and cardiovascular system[13]. However, the ability of researchers to definitively suggest specific interventions for the treatment of NAFLD is limited by numerous factors. It is still unclear exactly which form of exercise is superior, how long the exercise sessions should be, or if weight loss is required in the treatment of the disease. Studies which examine these questions are limited by the small number of patients studied. Very few studies examine a single intervention in isolation, as it is difficult to control for diet or exercise alone.
Additionally, it is challenging to determine exactly what aspect of an intervention led to the improvement in hepatic steatosis or metabolic derangements. Researchers, for the most part, rely on imaging or serology to determine the effects of their interventions, but these modalities are imperfect in evaluating the change or degree of steatosis. Biopsy, the gold standard in diagnosing NASH, is also difficult to obtain due to cost as well as health risks to the patient. Perhaps more studies should be performed in the population of living liver donors, since these patients require biopsy for donor evaluation, and the benefits of biopsy for both the future recipient and the donor likely outweigh the risks of biopsy. Many unknowns remain, and hopefully more research within this field will help in the creation of more evidence-based guidelines for physical activity as a treatment of NAFLD.
From the studies selected for this review, it seems that recommending moderate intensity exercise, which incorporates both aerobic and resistance components, is reasonable to treat NAFLD in able-bodied patients. While not discussed in depth in this review, encouraging healthy eating may offer additional health benefits for these patients. However, there undoubtedly is wide variation on the degree of functional mobility and ability for these patients to adhere to such a treatment plan. Physicians should also consider aspects which may limit a person’s ability to participate in lifestyle modification such as motivation, access to gym facilities and healthy food, and physical limitations. Studies of diabetic patients indicate that as the level of intensity of an exercise program increases, motivation and adherence diminish[43]. As the rates of obesity and morbid obesity continue to rise, a larger proportion of the NAFLD patient population will present challenges for treatment to physicians throughout the country. Acceleration and hybrid training, along with other low impact exercises may provide modest benefit for those patients limited by their body habitus or their poor cardiorespiratory fitness. Tackling this growing epidemic will likely require a strong multidisciplinary approach, combining physical activity, nutrition, and behavioral modification to develop a solution for this diverse patient population.
Nonalcoholic fatty liver disease (NAFLD) is becoming more prevalent throughout the world and increasingly problematic in terms of costs to the healthcare system and individual. While NAFLD carries with it the risk of progression to worsening hepatic steatosis and cirrhosis, the presence of NAFLD, a disease highly associated with the metabolic syndrome, also increases the risk of cardiovascular morbidity and mortality. The first line treatment for NAFLD incorporates both dietary modifications and exercise. However, there is a paucity of high-powered studies and substantial evidence to support this treatment as well as to prove the impact of this treatment on both the metabolic and cardiovascular derangements seen in NAFLD. The goal of this review is to examine the available evidence for exercise in NAFLD from 2010-2015 and to determine the efficacy of exercise to treat NAFLD and its concurrent disease states. The secondary aim of this review was to determine if any form of exercise or particular length or duration of exercise was more efficacious in treating this disease state and improving factors such as insulin sensitivity, hepatic steatosis, and visceral adiposity.
Numerous studies examine the effect of employing either resistance or aerobic exercise in the treatment of NAFLD. Researchers proposed modest exercise routines which can reasonably be completed by a substantial proportion of the NAFLD population. However, these programs may be difficult for patients with morbid obesity, advanced age, and other severe physical limitations. Newer studies have examined the benefit of low impact exercise, such as acceleration and hybrid training, in the treatment of NAFLD. While the results are modest, programs such as these may be employed to allow physically limited patients to achieve improvements in functional mobility as well as improvements in cardiovascular and metabolic health.
Both resistance and cardiovascular exercise regimens have been shown to demonstrate benefit in treating NAFLD. Newer forms of exercise, such as acceleration training and hybrid training, seem promising as well. Additionally, decreasing one’s sedentary time through increased physical activity even if the activity is low intensity may have health benefits for this patient population.
The studies reviewed support the benefits of lifestyle intervention on NAFLD and its resultant cardiovascular and metabolic disease. It is reasonable to recommend moderate intensity exercise which comprises of both aerobic and resistance exercise for patients.
NAFLD incorporates a range of disease from simple hepatic steatosis no nonalcoholic steatohepatitis. NAFLD is closely associated with the metabolic syndrome, and recent research has identified a strong association with cardiovascular disease, which represents the largest cause of mortality for patients with NAFLD.
In this invited manuscript, the authors aimed to conduct a systematic review evaluating published literature to assess the efficacy of exercise as a treatment for NAFLD and its effect on insulin resistance, dyslipidemia and amount of visceral adiposity. It seems to the authors a good manuscript, correctly developed with a suitable order, creates an awareness on the subject.
P- Reviewer: Sertoglu E S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Nobili V, Alkhouri N, Alisi A, Della Corte C, Fitzpatrick E, Raponi M, Dhawan A. Nonalcoholic fatty liver disease: a challenge for pediatricians. JAMA Pediatr. 2015;169:170-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1522] [Cited by in F6Publishing: 1564] [Article Influence: 120.3] [Reference Citation Analysis (1)] |
| 3. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 1244] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 4. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2221] [Article Influence: 170.8] [Reference Citation Analysis (0)] |
| 5. | Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600-606. [PubMed] [Cited in This Article: ] |
| 6. | Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 585] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 7. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1211] [Cited by in F6Publishing: 1315] [Article Influence: 146.1] [Reference Citation Analysis (1)] |
| 8. | Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373-379. [PubMed] [Cited in This Article: ] |
| 9. | Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | Than NN, Newsome PN. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239:192-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 11. | Takahashi Y, Sugimoto K, Inui H, Fukusato T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:3777-3785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 94] [Cited by in F6Publishing: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [PubMed] [Cited in This Article: ] |
| 13. | Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 14. | Gerber L, Otgonsuren M, Mishra A, Escheik C, Birerdinc A, Stepanova M, Younossi ZM. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36:772-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 866] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 16. | Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol (1985). 2011;111:1828-1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Johnson NA, George J. Fitness versus fatness: moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology. 2010;52:370-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Johnson NA, Keating SE, George J. Exercise and the liver: implications for therapy in fatty liver disorders. Semin Liver Dis. 2012;32:65-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Bacchi E, Negri C, Zanolin ME, Milanese C, Faccioli N, Trombetta M, Zoppini G, Cevese A, Bonadonna RC, Schena F. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care. 2012;35:676-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253-2262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 605] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 21. | Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, Harrison SA. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6:249-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 22. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] [Cited in This Article: ] |
| 23. | Jin YJ, Kim KM, Hwang S, Lee SG, Ha TY, Song GW, Jung DH, Kim KH, Yu E, Shim JH. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: analysis of biopsies of living liver donors. J Gastroenterol Hepatol. 2012;27:1341-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Sun WH, Song MQ, Jiang CQ, Xin YN, Ma JL, Liu YX, Ma L, Lin ZH, Li CY, Liu L. Lifestyle intervention in non-alcoholic fatty liver disease in Chengyang District, Qingdao, China. World J Hepatol. 2012;4:224-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31:661-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1019] [Cited by in F6Publishing: 975] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 26. | Hallsworth K, Thoma C, Moore S, Ploetz T, Anstee QM, Taylor R, Day CP, Trenell MI. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6:44-51. [PubMed] [Cited in This Article: ] |
| 27. | Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 28. | Oh S, Tanaka K, Tsujimoto T, So R, Shida T, Shoda J. Regular exercise coupled to diet regimen accelerates reduction of hepatic steatosis and associated pathological conditions in nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2014;12:290-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Hallsworth K, Fattakhova G, Hollingsworth K, Thoma C, Moore S, Taylor R, Day CP, Trenell MI. Resistance exercise improves liver lipid, fat oxidation and glucose control in adults with non-alcoholic fatty liver disease independent of weight loss. J Hepatol. 2011;54:S337. [DOI] [Cited in This Article: ] |
| 30. | Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, Kis O, Fliss-Isakov N, Izkhakov E, Halpern Z. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol. 2014;20:4382-4392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 89] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 31. | Webb M, Yeshua H, Zelber-Sagi S, Santo E, Brazowski E, Halpern Z, Oren R. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR Am J Roentgenol. 2009;192:909-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Kawaguchi T, Shiba N, Maeda T, Matsugaki T, Takano Y, Itou M, Sakata M, Taniguchi E, Nagata K, Sata M. Hybrid training of voluntary and electrical muscle contractions reduces steatosis, insulin resistance, and IL-6 levels in patients with NAFLD: a pilot study. J Gastroenterol. 2011;46:746-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Oh S, Shida T, Sawai A, Maruyama T, Eguchi K, Isobe T, Okamoto Y, Someya N, Tanaka K, Arai E. Acceleration training for managing nonalcoholic fatty liver disease: a pilot study. Ther Clin Risk Manag. 2014;10:925-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460-48; quiz 469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 287] [Article Influence: 22.1] [Reference Citation Analysis (1)] |
| 35. | Oh S, Shida T, Yamagishi K, Tanaka K, So R, Tsujimoto T, Shoda J. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology. 2015;61:1205-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 101] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 36. | Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR, Lu L, Pagadala MR, McCullough AJ, Flask CA. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2013;98:E1181-E1188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Johnson NA, Walton DW, Sachinwalla T, Thompson CH, Smith K, Ruell PA, Stannard SR, George J. Noninvasive assessment of hepatic lipid composition: Advancing understanding and management of fatty liver disorders. Hepatology. 2008;47:1513-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 973] [Cited by in F6Publishing: 928] [Article Influence: 66.3] [Reference Citation Analysis (1)] |
| 39. | Pugh CJ, Cuthbertson DJ, Sprung VS, Kemp GJ, Richardson P, Umpleby AM, Green DJ, Cable NT, Jones H. Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2013;305:E50-E58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, Green DJ, Green DJ, Cable NT, Jones H. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307:H1298-1306. [Cited in This Article: ] |
| 41. | Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 377] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 42. | O’Hagan C, De Vito G, Boreham CA. Exercise prescription in the treatment of type 2 diabetes mellitus : current practices, existing guidelines and future directions. Sports Med. 2013;43:39-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, Zanolin E, Schena F, Bonora E, Moghetti P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. 2013;58:1287-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |