Copyright
©2014 Baishideng Publishing Group Inc.
World J Hepatol. Aug 27, 2014; 6(8): 601-612
Published online Aug 27, 2014. doi: 10.4254/wjh.v6.i8.601
Published online Aug 27, 2014. doi: 10.4254/wjh.v6.i8.601
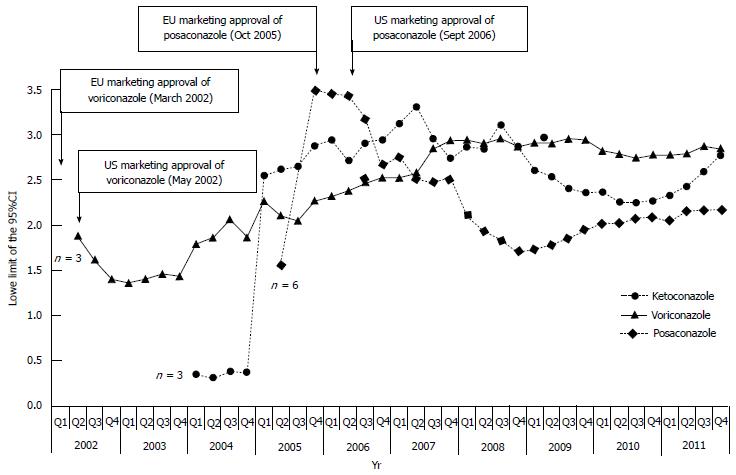
Figure 2 Cumulative time trend analysis of disproportionality (i.
e., lower limit of 95%CI) on overall liver injury for ketoconazole (recently restricted/suspended), voriconazole and posaconazole (the most recent antimycotics receiving marketing authorization in United States and Europe). Relevant regulatory milestones (i.e., marketing approvals) are indicated by arrows. The total number of cases is also indicated when disproportionality was calculated for the first time (the first point of the lower limit of the 95%CI is provided when at least 3 cases of interest were recorded); EU: Europe; US: United states.
- Citation: Raschi E, Poluzzi E, Koci A, Caraceni P, Ponti FD. Assessing liver injury associated with antimycotics: Concise literature review and clues from data mining of the FAERS database. World J Hepatol 2014; 6(8): 601-612
- URL: https://www.wjgnet.com/1948-5182/full/v6/i8/601.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i8.601