Copyright
©2014 Baishideng Publishing Group Inc.
World J Hepatol. Dec 27, 2014; 6(12): 880-893
Published online Dec 27, 2014. doi: 10.4254/wjh.v6.i12.880
Published online Dec 27, 2014. doi: 10.4254/wjh.v6.i12.880
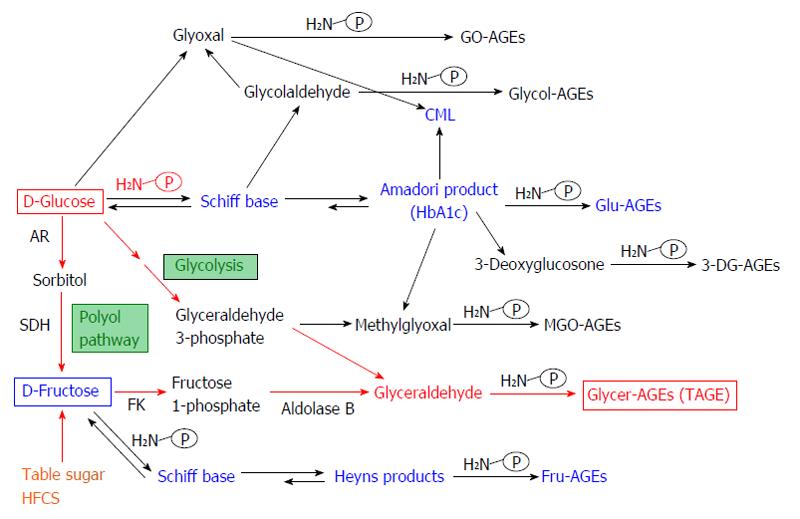
Figure 1 Alternative in vivo advanced glycation end-product synthesis routes.
Reducing sugars, such as glucose, fructose and glyceraldehyde, are known to react non-enzymatically with the amino groups of proteins to form reversible Schiff bases and Amadori product/Heyns products. These early glycation products undergo further complex reactions such as rearrangement, dehydration, and condensation to become irreversibly cross-linked, heterogeneous fluorescent derivatives termed advanced glycation end-products (AGEs). Glu-AGEs: Glucose-derived AGEs; Fru-AGEs: Fructose-derived AGEs; Glycer-AGEs: Glyceraldehyde-derived AGEs; Glycol-AGEs: Glycolaldehyde-derived AGEs; MGO-AGEs: Methylglyoxal-derived AGEs; GO-AGEs: Glyoxal-derived AGEs; 3-DG-AGEs: 3-deoxyglucosone-derived AGEs; CML: N-(carboxymethyl)lysine; P-NH2: A free amino residue; HbA1c: Hemoglobin A1c; TAGE: Toxic AGEs; HFCS: High-fructose corn syrup; AR: Aldose reductase; SDH: Sorbitol dehydrogenase; FK: Fructokinase.
- Citation: Takeuchi M, Takino JI, Sakasai-Sakai A, Takata T, Ueda T, Tsutsumi M, Hyogo H, Yamagishi SI. Involvement of the TAGE-RAGE system in non-alcoholic steatohepatitis: Novel treatment strategies. World J Hepatol 2014; 6(12): 880-893
- URL: https://www.wjgnet.com/1948-5182/full/v6/i12/880.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i12.880