Published online Aug 27, 2012. doi: 10.4254/wjh.v4.i8.242
Revised: August 6, 2012
Accepted: August 23, 2012
Published online: August 27, 2012
AIM: To assess vitamin D in hepatitis C patients and its relationship to interleukin (IL)-23, IL-17, and macrophage chemoattractant protein-1 (MCP-1).
METHODS: The study was conducted on 50 Egyptian hepatitis C virus (HCV) genotype number IV-infected patients and 25 age- and gender-matched healthy subjects. Venous blood samples were obtained. Samples were allowed to clot and sera were separated by centrifugation and stored at -20 °C. A 25 hydroxy vitamin D assay was carried out using solid phase RIA. A 1,25 dihydroxy vitamin D assay was carried out using a commercial kit purchased from Incstar Corporation. IL-17 and -23 and MCP-1 were assayed by an enzyme immunoassay. Quantitative and qualitative polymerase chain reaction for HCV virus were done by TaqMan technology. Only HCV genotype IV-infected subjects were included in the study. The mean ± SD were determined, a t-test for comparison of means of different parameters was used. Correlation analysis was done using Pearson’s correlation. Differences among different groups were determined using the Kruskal-Wallis test.
RESULTS: The mean vitamin D level in HCV patients (group I) was 15 ± 5.2 ng/mL while in control (group II) was 39.7 ± 10.8. For active vitamin D in group I as 16.6 ± 4.8 ng/mL while in group II was 41.9 ± 7.9. IL-23 was 154 ± 97.8 in group I and 6.7 ± 2.17 in group II. IL-17 was 70.7 ± 72.5 in cases and 1.2 ± 0.4 in control. MCP-1 was 1582 ± 794.4 in group I and 216.1 ± 5.38 in group II. Vitamin D deficiency affected 72% of HCV-infected patients and 0% of the control group. Vitamin D insufficiency existed in 28% of HCV-infected patients and 12% of the control group. One hundred percent of the cirrhotic patients and 40% of non cirrhotic HCV-infected patients had vitamin D deficiency. IL-23, IL-17, and MCP-1 were markedly increased in HCV-infected patients in comparison to controls.A significant negative correlation between vitamin D and IL-17 and -23 and MCP-1 was detected. HCV-infected males and females showed no differences with respect to viral load, vitamin D levels, IL-17, IL-23 and MCP-1. The viral load was negatively correlated with vitamin D and active vitamin D (P = 0.0001 and P = 0.001, respectively), while positively correlated with IL-23, IL-17, and MCP-1. We classified the patients according to sonar findings into four groups. Group Ia with bright hepatomegaly and included 14 patients. Group Ib with perihepatic fibrosis and included 11 patients. Group Ic with liver cirrhosis and included 11 patients. Group Id with hepatocellular carcinoma (HCC) and included 14 patients. Vitamin D and active vitamin D were shown to be lower in cirrhotic patients and much lower in patients with HCC, and this difference was highly significant (P = 0.0001). IL-17 and -23 and MCP-1 were higher in advanced liver disease) and the differences were highly significant (P = 0.0001).
CONCLUSION: Whether the deficiency of vitamin D is related to HCV-induced chronic liver disease or predisposing factor for higher viral load is a matter of debate.
- Citation: El Husseiny NM, Fahmy HM, Mohamed WA, Amin HH. Relationship between vitamin D and IL-23, IL-17 and macrophage chemoattractant protein-1 as markers of fibrosis in hepatitis C virus Egyptians. World J Hepatol 2012; 4(8): 242-247
- URL: https://www.wjgnet.com/1948-5182/full/v4/i8/242.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i8.242
Vitamin D is a critical regulator of immunity, playing a role in both innate and cell-mediated immune responses. Vitamin D suppresses the production of T helper (Th)1 cytokines, such as interferon-γ (IFN-γ) and interleukin (IL)-2, and consequently leads to enhanced production of Th2 cytokines, such as IL-4 and -5, thus potentially promoting humoral immune responses. Vitamin D also promotes innate immunity by directly inducing the gene expression of antimicrobial peptides (cathelicidin and β-defensin 2) in various human cell types[1-4].
Vitamin D deficiency has been shown to be associated with several immune-mediated diseases, and susceptibility to infection and cancer. In fact, a 25(OH)D concentration < 50 nmol/L (20 ng/mL) is an indication of vitamin D deficiency, whereas a 25(OH)D concentration of 51-74 nmol/L (21-29 ng/mL) is considered to indicate insufficiency[5,6]
IL-23, in conjunction with IL-6 and transforming growth factor β (TGF-β), stimulates the differentiation of Th17 cells with subsequent production of IL-17[7]. IL-17 is a cytokine that acts as a potent mediator in delayed-type reactions by increasing chemokine production in various tissues to recruit monocytes and neutrophils to the site of inflammation, similar to IFN-γ. IL-17 acts synergistically with tumor necrosis factor (TNF) and IL-1[8].
Chronic hepatic cirrhotic patients with genotype 1 have low 25(OH)D serum levels. Low vitamin D is linked to severe fibrosis and a low sustained virologic response (SVR) on IFN-based therapy[9,10].
There is interesting preliminary data that indicate that 1,25(OH)2D3 suppresses Th17 driven cytokine responses, induces Treg cells, induces IL-4 production (Th2) and enhances natural killer T-cell function; differentiation and maturation of B cells is also inhibited. In addition, treatment with vitamin D receptor (VDR) agonists inhibits the T-cell production of IL-17. Furthermore, IL-17 production is sustained by IL-23, an IL-12 family member, the latter of which is strongly inhibited by VDR agonists[11].
Also, 1,25(OH)2D3 has been shown to inhibit macrophage chemoattractant protein-1 (MCP-1)-driven inflammatory process by blocking nuclear factor-κB activation. MCP-1 is expressed in injury and inflammation and leads to direct macrophage recruitment[12].
Hepatitis C virus (HCV) is remarkably efficient at establishing persistent infections, suggesting that HCV has evolved one or more strategies aimed at evading the host immune response. T cell responses, including IFN-γ production, are severely suppressed in patients with chronic HCV infections[13].
Aim of the study: To assess the relationship between vitamin D and markers of inflammation in HCV infected patients and measure the degree of this relation to viral load and degree of fibrosis.
The study approved by Ethical Committee. The study included 50 patients with HCV-related chronic liver disease with a minimum duration of 7 years (group I), who attended the Hepatology Outpatient Clinic, Endemic Disease Hospital, Faculty of Medicine, Cairo University.
Collection of patients required 4 mo. Inclusion criteria were based on previous history of liver disease with HCV infection of both sexes, whether new patients or under follow up were included. Isolated HBV or coinfection with HBV and HIV infected patients were excluded.
Group I included 36 males (72%) and 14 females (28%), ranging in age from 30-65 years, with a mean age of 47.5 years. Twenty-five age- and gender-matched healthy subjects were included as a control group (group II). The controls had liver functions and abdominal U/S and test for HCV antibodies which were all normal. Informed consent was obtained from the patients and controls regarding all the procedures done.
All patients were subjected to thorough history-taking and a clinical examination. Abdominal ultrasonography was performed on all patients, and according to the results, patients were classified into 4 subgroups as follows: 14 patients with bright hepatomegly; 11 patients with perihepatic fibrosis; 11 patients with hepatic cirrhosis; and14 patients with hepatocellular carcinoma (HCC) and cirrhosis.
Venous blood samples were obtained after overnight fasting from all patients. Samples were allowed to clot and sera were separated by centrifugation and stored at -20 °C.
A 25 hydroxy vitamin D assay was carried out using a commercial kit purchased from (Medgenix Diagnostics S.A. Zoning Industrial. B-6220 Fleurus, Belgium; Mawer, 1980) using solid phase RIA. A 1,25 dihydroxy vitamin D assay was carried out using a commercial kit purchased from Incstar Corporation (Stillwater, MN USA; Hollis, 1986). IL-17 and -23 and MCP-1 were assayed by an enzyme immunoassay (Biosource Europe S.A). Quantitative and qualitative PCR for HCV virus were done by TaqMan technology. Only HCV genotype IV-infected subjects were included in the study.
SPSS (version 15) was used for statistic measures of this study. The mean ± SD were determined, a t-test for comparison of means of different parameters was used. Correlation analysis was done using Pearson’s correlation. Differences among different groups were determined using the Kruskal-Wallis test.
Vitamin D deficiency, defined as a serum vitamin D level < 20 ng/mL, was present in 36 patients (72%) and none (0%) of the control group. Vitamin D insufficiency (20-29 ng/mL) existed in 14 (28%), HCV-infected patients and 3 (12%) subjects in the control group. Furthermore, 25 (100%) cirrhotic patients had vitamin D deficiency and 10 (40%) non-cirrhotic HCV-infected cases. Table 1 shows the laboratory data of the study groups and demonstrates a statistically significant difference with respect to vitamin D and its active form, IL-23, and IL-17 between both groups. The viral load mean was 128 000 ± 28 000 IU/mL.
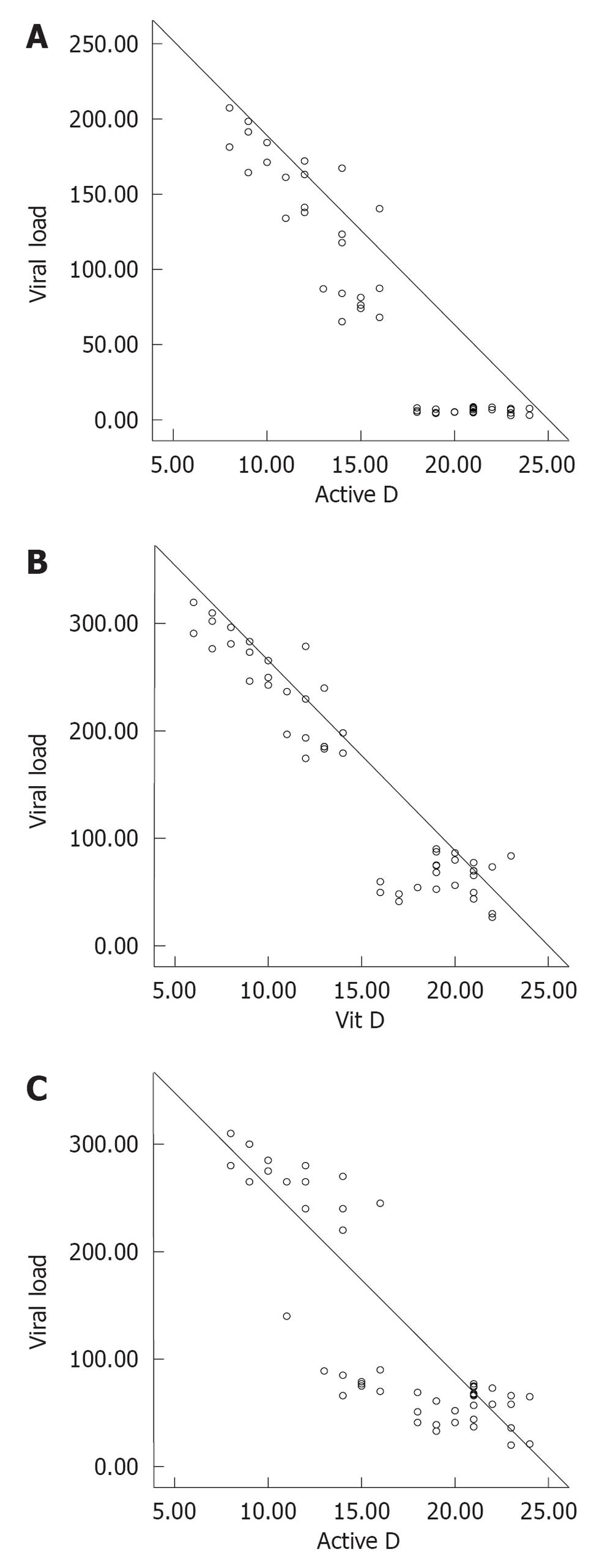
Table 2 demonstrates the correlation between different parameters in HCV-infected subjects and controls. There was significant negative correlation between vitamin D and viral load, IL-23, IL-17 and MCP-1. Meanwhile there was a positive correlation between viral load and IL-17, IL-23 and MCP-1. Table 3 shows the studied parameters in HCV-infected patients when classified into 2 subgroups according to gender. Figure 1 show correlations between vitamin D and IL-23, IL-17, and viral load, respectively. Table 4 demonstrates the laboratory data in the four subgroups of HCV-infected patients. Vitamin D and active vitamin D were shown to be lower in cirrhotic patients and much lower in patients with HCC, and this difference was highly significant (P = 0.0001). IL-17 and -23 and MCP-1 were higher in advanced liver disease) and the differences were highly significant (P = 0.0001)
| Items | R | P value |
| Vit D, viral load | -0.84 | 0.000 |
| Active D, viral load | -0.846 | 0.000 |
| Vit D and IL-23 | -0.776 | 0.000 |
| Active D and IL-23 | -0.801 | 0.000 |
| Vit D and IL-17 | -0.665 | 0.000 |
| Active D and IL-17 | -0.679 | 0.000 |
| IL-17 and viral load | 0.951 | 0.000 |
| IL-23 and viral load | 0.922 | 0.000 |
| MCP-1 and viral load | 0.94 | 0.000 |
| MCP-1 and vitamin D | -0.94 | 0.000 |
| MCP-1 and active D | -0.92 | 0.000 |
| Item | Male group (n = 27) | Female group (n = 23) | P value |
| Vit D (ng/mL) | 15.0 ± 5.12910 | 15.0 ± 5.6 | 1 |
| Active vit D (ng/mL) | 16.6 ± 4.5 | 16.6 ± 5.3 | 0.96 |
| Viral load (IU/mL) | 126.8 ± 98.6 | 129.3 ± 102.6 | 0.93 |
| IL-23 (ng/mL) | 152.4 ± 96.6 | 156.1 ± 101.3 | 0.92 |
| IL-17 (ng/mL) | 69.8 ± 69.2 | 71.9 ± 77.6 | 0.9 |
| MCP-1 (ng/mL) | 1575.7 ± 765.4 | 1589.4 ± 844.5 | 0.9 |
| Item | Group Ia (bright hepatomegaly) (n = 14) | Group Ib (perihepatic fibrosis) (n = 11) | Group Ic (liver cirrhosis) (n = 11) | Group Id (HCC) (n = 14) | Normal (n = 25) | P value |
| IL-17 (ng/mL) | 7.6 | 5.1 | 115.9 | 150.3 | 1.26 | 0.000 |
| IL-23 (ng/mL) | 76.8 | 51.2 | 259.3 | 225.9 | 6.7 | 0.000 |
| Vit D (ng/mL) | 19.8 | 19.4 | 10.9 | 9.7 | 39.1 | 0.000 |
| Active vit D (ng/mL) | 20.6 | 21 | 13 | 11.7 | 41.1 | 0.000 |
| Viral load (IU/mL) | 66.3 | 42.4 | 165.1 | 231.1 | 0 | 0.000 |
| MCP-1 (ng/mL) | 910.3 | 838.8 | 2090.9 | 2448 | 237.34 | 0.000 |
The liver plays a central role in vitamin D metabolism. Vitamin D inadequacy is common in non-cholestatic chronic liver diseases and correlates with disease severity. The current study showed a significant reduction of vitamin D and its active metabolites in HCV genotype 4-infected patients compared to healthy controls. This reduction was more prevalent and severe in cirrhotic vs non-cirrhotic patients. This is consistent with previous studies done on patients with genotype 1, which showed that vitamin D deficiency is universal (92%) among patients with chronic liver disease, and at least one-third of the patients have severe vitamin D deficiency[14-16].
Our results showed that IL-23 and -17 were markedly increased in HCV-infected patients in comparison to controls. Regulation of Th1 and Th17 responses in HCV-infected individuals was studied, and it was reported that TGF-β and IL-6 promote differentiation of naive murine CD4+ T cells into IL-17-secreting Th17 cells. In addition, it has been reported that other innate cytokines, including IL-1, IL-23, TNF-α, and IL-21, in different combinations or with TGF-β, are also involved in differentiation, amplification, or stabilization of the Th17 phenotype[17,18].
Our study reported that there is a significant negative correlation between vitamin D and IL-17 and -23. Previous studies on mice showed that vitamin D is a strong inhibitor of Th17 polarization and Th17 cytokine expression of splenic CD4+ T cells. Furthermore, Th17 differentiation from naïve T cells was affected by vitamin D. These data implicate a regulatory mechanism on Th17 cells by vitamin D, through the reduction of RORgt expression[19].
The effect of vitamin D on the behavior of Th17 cells was investigated in different diseases and it was found that vitamin D suppressed the expression of IL-17 and -23[20-23].
We reported a positive correlation between IL-23 and -17 with viral load, a finding which further support our suggestion regarding the link between vitamin D and both IL-17 and -23 in immune regulation in HCV genotype IV-related chronic liver disease. These findings may support our suggestion that increased IL-17 and -23 could be, at least in part, involved in the role of vitamin D in the immune response in HCV genotype IV-related liver disease and explain how vitamin D deficiency plays a role in increasing liver fibrosis.
Our results revealed HCV-infected males and females had no differences with respect to vitamin D levels. In contrast with our results, Arteh et al[24] who reported that African American females with chronic liver disease are at higher risk of vitamin D deficiency.
Our study showed that the viral load mean value was 1.28 × 105± 28 × 103 IU/mL. A significant negative correlation was reported between vitamin D and active vitamin D and viral load (P = 0.0001 and P = 0.001, respectively).
Vitamin D is an important immune modulator and preliminary data indicated an association between vitamin D deficiency and SVR rates in HCV as reduced 25-hydroxyvitamin D levels and CYPB27-1260 promotor polymorphism with reduced 1,25-dihydroxyvitamin D levels are associated with failure to achieve SVR in HCV genotypes 1-, 2-, and 3-infected patients[9,25]. Our HCV patients with genotype IV need further follow up to confirm the effect of vitamin D deficiency on their responses to treatment.
There was a significant increase in level of MCP-1 in our patients with all grades of hepatic affection in comparison to controls. Similar results were reported by Camps et al[26]. However, Panasiuk et al[27] reported a decrease in the MCP-1 level in liver cirrhosis in comparison to the controls and did not reflect any inflammatory process in liver cirrhosis. More studies are needed to explore this point of controversy.
Our results also revealed a significant negative correlation between vitamin D and MCP-1. This supports the role of decreased vitamin D in inflammation and fibrosis. No previous work in hepatic patients studied this relationship. However, Zehnder et al[28] reported that reduction of the vitamin D hormonal system in kidney disease was associated with increased renal inflammation and fibrosis. Zehnder et al[28] reported a significant negative correlation between vitamin D and MCP-1. Logistic regression analysis with urinary MCP-1 as a binary outcome showed that a 10-unit increase in serum 1,25(OH)2D or 25OHD resulted in lower renal inflammation[28].
On classifying HCV-infected patients according to sonar finding into four groups, vitamin D and active vitamin D were shown to be lower in cirrhotic patients and much lower in patients with HCC, and this difference was highly significant (P = 0.0001). IL-17 and -23 and MCP-1 were higher in advanced liver disease) and the differences were highly significant (P = 0.0001). These findings are concomitant with previous results which indicate that vitamin D inadequacy is common in non-cholestatic chronic liver diseases and correlates with disease severity[14]. The difference in viral load among these groups may explain in part the difference in levels of inflammatory cytokines.
In conclusion, vitamin D deficiency is prevalent in HCV genotype IV-infected patients and viral load is negatively correlated to vitamin D. Whether or not this deficiency is related to HCV-induced chronic liver disease or predisposing factor for higher viral load is a matter of debate. In view of the immune function of vitamin D, vitamin D status may be assessed and supplements may be considered to achieve a SVR with IFN-based therapy. The negative correlation between vitamin D and IL-23 and -17 and MCP-1 may highlight, at least in part, how these cytokines might be involved with vitamin D in immune responses in HCV genotype IV-related liver disease and may explain how vitamin D deficiency plays a role in increasing liver fibrosis.
Special thanks to workers at the Hepatology Clinic of Kasr El Eini-Cairo University for their help during this research.
Vitamin D receptor (VDR) is found in significant concentrations in the T lymphocyte and macrophage populations. However, the highest concentration of VDR is in the immature immune cells of the thymus and the mature CD-8 T lymphocytes.
This study highlights the relationship between interleukin (IL)-23, IL-17 and macrophage chemoattractant protein-1 (MCP-1) with vitamin D in patients with hepatitis C virus (HCV).
In view of the immune function of vitamin D, vitamin D status may be assessed and supplements may be considered to achieve a sustained virologic response with interferon-based therapy. The negative correlation between vitamin D and IL-23 and -17 and MCP-1 may highlight, at least in part, how these cytokines might be involved with vitamin D in immune responses in HCV genotype IV-related liver disease and may explain how vitamin D deficiency plays a role in increasing liver fibrosis.
IL-23, IL-17 and MCP-1 can be used as markers of degree of liver fibrosis. Vitamin D supplements may improve immune response and delays fibrosis induced by HCV.
Authors studied the relation between serum vitamin D levels and HCV related liver disease. They detected a strong correlation with severity fibrosis, treatment response and cytokine levels which has been also shown previously.
Peer reviewers: Yasemin Hatice Balaban, Professor, Hacettepe University, Medical Faculty Internal Medicine Department, Gastroenterology Unit, Ankara 06100, Turkey; Ajith TA, PhD, Assistant Professor of Biochemistry, Department of Biochemistry, Amala Institute of Medical Sciences, Amala Nagar, Thrissur, 680 555, India
S- Editor Jia F L- Editor A E- Editor Zheng XM
| 1. | Shirakawa AK, Nagakubo D, Hieshima K, Nakayama T, Jin Z, Yoshie O. 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J Immunol. 2008;180:2786-2795. [PubMed] [Cited in This Article: 1] |
| 2. | Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579-2585. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 562] [Cited by in RCA: 529] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 3. | Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922-932. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 327] [Cited by in RCA: 310] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Muller K, Svenson M, Bendtzen K. 1 alpha,25-Dihydroxyvitamin D3 and a novel vitamin D analogue MC 903 are potent inhibitors of human interleukin 1 in vitro. Immunol Lett. 1988;17:361-365. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert Rev Clin Immunol. 2009;5:693-702. [PubMed] [Cited in This Article: 1] |
| 6. | Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S-1086S. [PubMed] [Cited in This Article: 1] |
| 7. | McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445-453. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 500] [Cited by in RCA: 528] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 8. | Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888-898. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 1059] [Cited by in RCA: 1065] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 9. | Petta S, Cammà C, Scazzone C, Tripodo C, Di Marco V, Bono A, Cabibi D, Licata G, Porcasi R, Marchesini G. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158-1167. [PubMed] [DOI] [Full Text] [Cited in This Article: 2] [Cited by in Crossref: 305] [Cited by in RCA: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 10. | Mouch SA, Fireman Z, Jarchovsky J, Assy N. Vitamin D supplement improve SVR in chronic hepatitis C (genotype 1) Naïve patients treated with Peg interferon and ribavirin. EASL 45th Annual Meeting of European Association for the Study of the Liver;. 2010;Apr 14-18; Vienna, Austria. [Cited in This Article: 1] |
| 11. | Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139-145. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 978] [Cited by in RCA: 967] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 12. | Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495-504. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 109] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Eisen-Vandervelde AL, Waggoner SN, Yao ZQ, Cale EM, Hahn CS, Hahn YS. Hepatitis C virus core selectively suppresses interleukin-12 synthesis in human macrophages by interfering with AP-1 activation. J Biol Chem. 2004;279:43479-43486. [PubMed] [Cited in This Article: 1] |
| 14. | Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5:513-520. [PubMed] [DOI] [Full Text] [Cited in This Article: 2] [Cited by in Crossref: 138] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Bouillon R, Auwerx J, Dekeyser L, Fevery J, Lissens W, De Moor P. Serum vitamin D metabolites and their binding protein in patients with liver cirrhosis. J Clin Endocrinol Metab. 1984;59:86-89. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Duarte MP, Farias ML, Coelho HS, Mendonça LM, Stabnov LM, do Carmo d Oliveira M, Lamy RA, Oliveira DS. Calcium-parathyroid hormone-vitamin D axis and metabolic bone disease in chronic viral liver disease. J Gastroenterol Hepatol. 2001;16:1022-1027. [PubMed] [Cited in This Article: 1] |
| 17. | Rowan AG, Fletcher JM, Ryan EJ, Moran B, Hegarty JE, O'Farrelly C, Mills KH. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J Immunol. 2008;181:4485-4494. [PubMed] [Cited in This Article: 1] |
| 18. | Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636-2649. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 269] [Cited by in RCA: 286] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Mus AM, van Hamburg JP, Asmawidjaja P, Hazes JMW, van Leeuwen H, Boon L, Colin E. Vitamin D suppresses Th17 cytokines via down regulation of RORgammat and NFATC2 and by differential regulation of GATA3. Arthritis Rheum. 2010;62 Suppl 10:38. [Cited in This Article: 1] |
| 20. | van Hamburg JP, Asmawidjaja PS, Davelaar N, Cornelissen FC, Mus AMC, Bakx PAGM, Colin EM, van Leeuwen H, Hazes JMW, Dolhain RJEM. Vitamin D suppresses the pathogenic behaviour of primary Th17 cells from patients with early rheumatoid arthritis. Ann Rheum Dis. 2011;70:A47. [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramírez P, Martin-Loeches I, Varillas D, Gallegos MC, Serón C. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: 1] [Cited by in Crossref: 254] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Zold E, Szodoray P, Kappelmayer J, Gaal J, Csathy L, Barath S, Gyimesi E, Hajas A, Zeher M, Szegedi G. Impaired regulatory T-cell homeostasis due to vitamin D deficiency in undifferentiated connective tissue disease. Scand J Rheumatol. 2010;39:490-497. [PubMed] [Cited in This Article: 1] |
| 23. | Bartosik-Psujek H, Tabarkiewicz J, Pocinska K, Stelmasiak Z, Rolinski J. Immunomodulatory effects of vitamin D on monocyte-derived dendritic cells in multiple sclerosis. Mult Scler. 2010;16:1513-1516. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624-2628. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 254] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (2)] |
| 25. | Lange CM, Bojunga J, Ramos-Lopez E, von Wagner M, Hassler A, Vermehren J, Herrmann E, Badenhoop K, Zeuzem S, Sarrazin C. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J Hepatol. 2011;54:887-893. [PubMed] [Cited in This Article: 1] |
| 26. | Camps J, Marsillach J, Rull A, Alonso-Villaverde C, Joven J. Interrelationships between paraoxonase-1 and monocyte chemoattractant protein-1 in the regulation of hepatic inflammation. Adv Exp Med Biol. 2010;660:5-18. [PubMed] [DOI] [Full Text] [Cited in This Article: 1] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 27. | Panasiuk A, Zak J, Kasprzycka E, Janicka K, Prokopowicz D. Blood platelet and monocyte activations and relation to stages of liver cirrhosis. World J Gastroenterol. 2005;11:2754-2758. [PubMed] [Cited in This Article: 1] |
| 28. | Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV, Raymond NT, Howie AJ, Cockwell P, Stewart PM. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74:1343-1353. [PubMed] [Cited in This Article: 3] |