Copyright
©The Author(s) 2024.
World J Hepatol. Nov 27, 2024; 16(11): 1306-1320
Published online Nov 27, 2024. doi: 10.4254/wjh.v16.i11.1306
Published online Nov 27, 2024. doi: 10.4254/wjh.v16.i11.1306
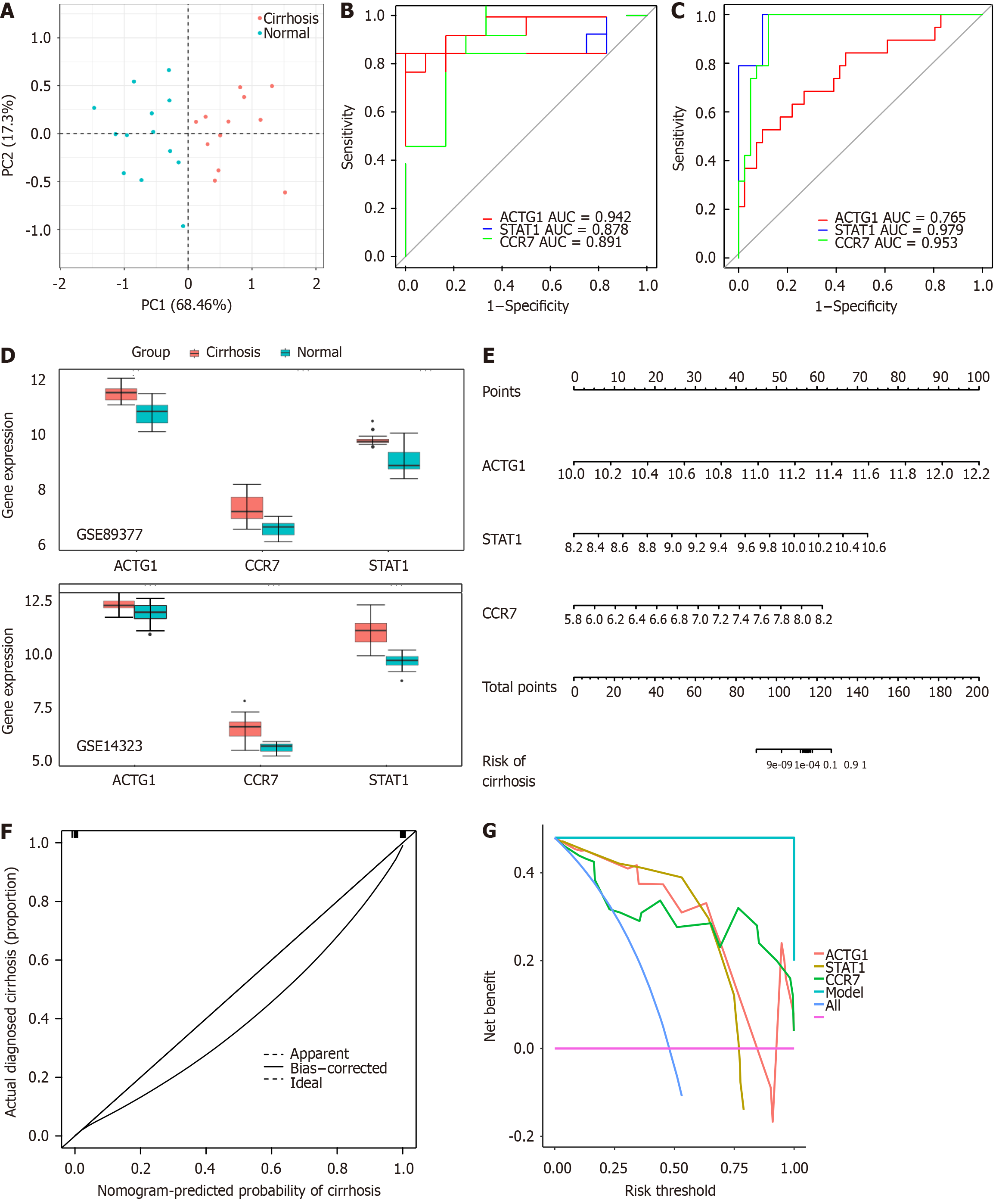
Figure 3 Assessment and validation of the diagnostic efficacy of biomarkers.
A: Principal component analysis was performed to analyze whether the three biomarkers could distinguish between control and cirrhotic samples; B: Receiver operating characteristic (ROC) curves of the biomarkers in the GSE89377 dataset; C: ROC curves of the biomarkers in the GSE14323 dataset; D: Expression of the biomarkers in the GSE89377 and GSE14323 datasets; E: A nomogram based on the biomarkers; F: Calibration curve of the nomogram; G: Decision curve analysis of the nomogram. AUC: Area under curve.
- Citation: Luo JY, Zheng S, Yang J, Ma C, Ma XY, Wang XX, Fu XN, Mao XZ. Development and validation of biomarkers related to anoikis in liver cirrhosis based on bioinformatics analysis. World J Hepatol 2024; 16(11): 1306-1320
- URL: https://www.wjgnet.com/1948-5182/full/v16/i11/1306.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i11.1306