Published online May 27, 2022. doi: 10.4254/wjh.v14.i5.885
Peer-review started: December 22, 2021
First decision: February 8, 2022
Revised: February 20, 2022
Accepted: April 25, 2022
Article in press: April 25, 2022
Published online: May 27, 2022
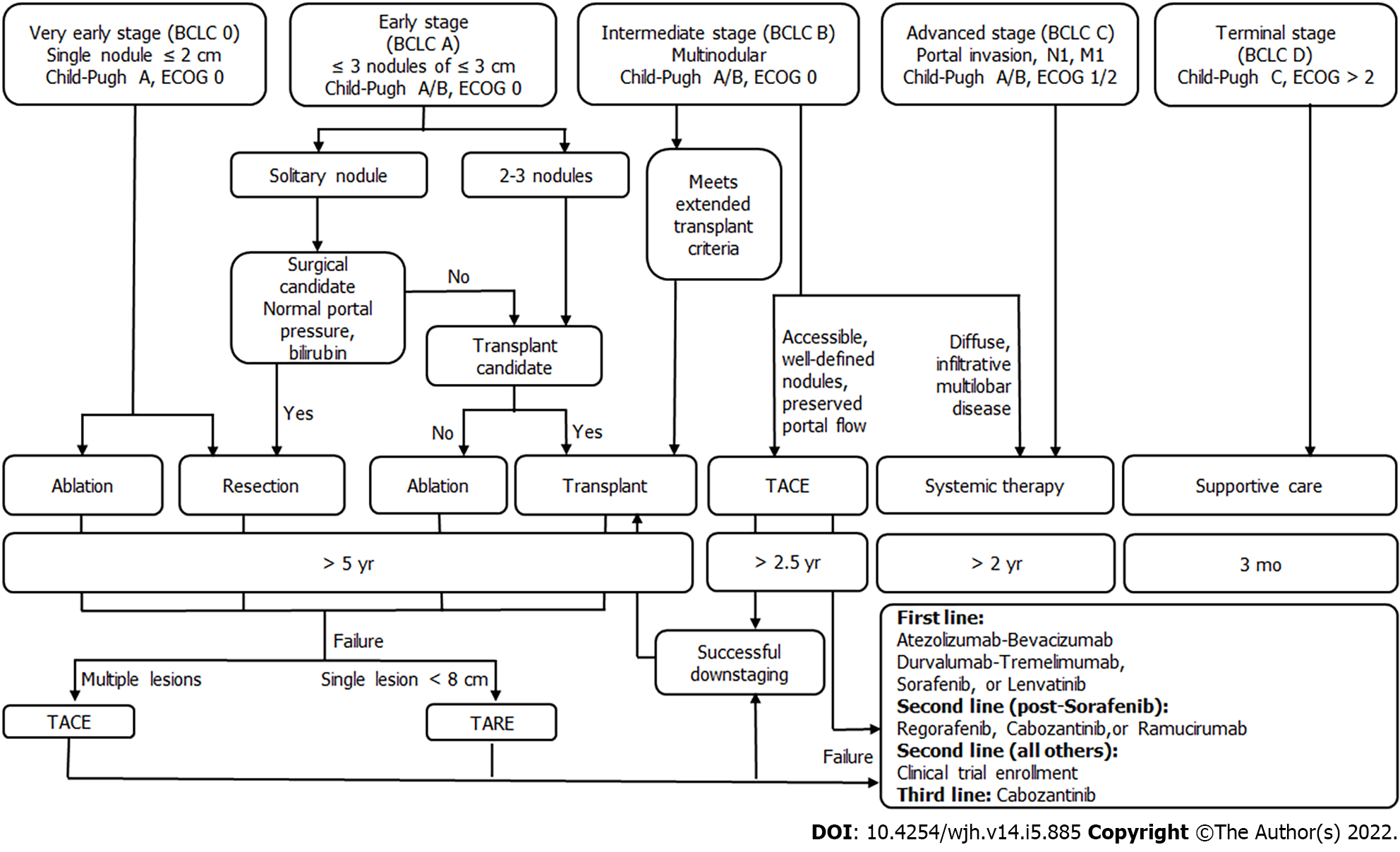
Hepatocellular carcinoma (HCC) is the most common cause of liver malignancy and the fourth leading cause of cancer deaths universally. Cure can be achieved for early stage HCC, which is defined as 3 or fewer lesions less than or equal to 3 cm in the setting of Child-Pugh A or B and an ECOG of 0. Patients outside of these criteria who can be down-staged with loco-regional therapies to resection or liver transplantation (LT) also achieve curative outcomes. Traditionally, surgical resection, LT, and ablation are considered curative therapies for early HCC. However, results from recently conducted LEGACY study and DOSISPHERE trial demonstrate that transarterial radio-embolization has curative outcomes for early HCC, leading to its recent incorporation into the Barcelona clinic liver criteria guidelines for early HCC. This review is based on current evidence for curative-intent loco-regional therapies including radioembolization for early-stage HCC.
Core Tip: Accepted curative modalities for early hepatocellular carcinoma (HCC) include resection, liver transplant, and loco-regional therapies. In this manuscript, we review the curative-intent loco-regional therapies including recent evidence from the LEGACY study and DOSISPHERE trial demonstrating a curative role for transarterial radio-embolization in early HCC.
- Citation: Zane KE, Nagib PB, Jalil S, Mumtaz K, Makary MS. Emerging curative-intent minimally-invasive therapies for hepatocellular carcinoma. World J Hepatol 2022; 14(5): 885-895
- URL: https://www.wjgnet.com/1948-5182/full/v14/i5/885.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i5.885
Hepatocellular carcinoma (HCC) is the most common cause of liver malignancy and the fourth leading cause of cancer death across the globe[1]. Curative outcomes can be achieved for early stage HCC, which is defined using the Barcelona clinic liver criteria (BCLC) as 3 or fewer lesions less than or equal to 3 cm in diameter with preserved liver function and functional status. Patients with intermediate or advanced HCC who can be down-staged with loco-regional therapies (LRT) to resection or tran
In early HCC, resection and LT are often preferred over ablation when possible. However, many patients are not surgical candidates, whether due to medical comorbidities, inability to tolerate anesthesia, or tumor location. Additional drawbacks to surgical approaches include elevated post-operative morbidity and mortality rates[2,3], life-long immunosuppression in the case of LT[4], and high recurrence rates with resection[5]. Fortunately, loco-regional therapies such as radiofrequency ablation (RFA) make curative treatment possible for these patients. Studies demonstrating comparable overall survival and recurrence rates for ablation are now over a decade old, and ablation as a curative therapy has been present in the National Comprehensive Cancer Network (NCCN) guidelines since 2017[6]. However, recent work demonstrates that transarterial radio-embolization (TARE) is an effective, safe, and curative treatment option for early HCC.
This review discusses the evidence for curative outcomes in early HCC using LRT including ablation, TARE, transarterial chemoembolization (TACE), and combination therapy. We further review the role of these therapies in down-staging and bridging with curative intent for LT or resection.
While cure is the ultimate goal in the treatment of HCC, it is not always apparent what defines a curative-intent therapy. Cure for HCC using various modalities including LT, resection and ablation is reported in the form of overall survival and recurrence. When curative LT outcomes are considered for HCC, overall survival is > 70% at 5 years[7], with a recurrence rate of 6%-15%[8]. For resection, overall survival is 50%-70% at 5 years[3,9] and recurrent HCC is seen in > 60% of patients at 5 years[5,10]. Notably, these outcomes may be influenced by differing criteria between surgical and locoregional therapy candidacy; for example, surgical candidates are typically without significant portal hypertension[11]. For ablation, overall survival is around 60% at 5 years with a local recurrence rate of 3%-22% at 5 years in lesions up to 5 cm[12,13]. All three therapies are considered potentially curative in appropriate patients, and thus establish a standard for outcomes necessary to be considered curative (Table 1).
| Modality | Overall survival at 5 yr (%) | Local tumor progression at 2 yr (%) | Local tumor progression at 5 yr (%) | Disease-free survival at 5 yr (%) |
| Transplant | ≥ 70[21] | NDA | Cumulative recurrence < 15[22] | > 70[23] |
| Resection | 60-80[24-26] | NDA | Resection margin recurrence 1-7[27-29] | 38-54[26,27,30] |
| Ablation ≤ 3 cm | 44-69[13,25,31] | 2-16[28,29,32] | 9.7-22[13,33,34] | 14-46[25,27] |
| TARE ≤ 3 cm | 75[35] | 2.4-6.1[36,37] | NDA | NDA |
| Ablation ≤ 5 cm | 49-72[27,38,39] | 6-9[40,41] | 3-14[12,31,40] | 50-59[27,40] |
| TARE ≤ 5 cm | 57[35] | 6.1-10[37,42] | 28 for ≤ 5 cm[35] | NDA |
Of note, the modified Response Evaluation Criteria in Solid Tumors (mRECIST) assessment of the radiologic response to LRT validates the use of tumor response rate as a surrogate outcome for survival[14]. Generally speaking, tumor response rate utilizes established imaging criteria to group patients into non-responders, partial responders, and complete responders[15]. The commonly reported objective response rate (ORR) is the combination of partial and complete responders over all subjects. As might be expected, complete response is associated with the greatest improvement in outcomes[16].
Loco-regional therapies also play a neo-adjuvant role in the treatment of HCC. They can be used to bridge patients to definitive therapy with LT or can down-stage HCC to meet transplant criteria. Using LRT, down-staging is successful in approximately half of patients, regardless of whether TARE or TACE is used[17]. Importantly, bridging and down-staging do not worsen LT outcomes in terms of overall survival or recurrence[18-20].
The most common ablation techniques for HCC are radiofrequency (RFA), microwave ablation (MWA), and cryoablation. In early stage HCC with preserved liver functions (Child-Pugh A/B), this is a potentially curative modality for patients who are not candidates for surgery or resection. In radiofrequency (RFA) and microwave ablation (MWA), probes are placed percutaneously into the tumor so that thermal energy may be used to directly induce tumor necrosis. In cryoablation, cold gas is delivered through hollow needles into the tumor tissue and frozen, inducing cell death[43]. For very early (BCLC 0) and early-stage (BCLC A) HCC patients with preserved liver function (Child-Pugh A/B), RFA and MWA offers survival outcomes comparable to resection despite lower baseline liver function[44-46]. These early-stage HCC patients are often disqualified from surgery by significant comorbidities, portal hypertension, poor hepatic function, intolerance to general anesthesia, or high-risk lesion location[47]. The use of ablation is limited by tumor location near central biliary structures, gallbladder, stomach, or sub-diaphragmatically given risk of unintended damage to these structures, as well as concern for the rare possibility of tumor tract seeding in case of sub-capsular tumors[35]. Additionally, ablation near large vascular structures decreases the ablative power as a heat sink effect from fluid flow draws thermal energy away from the target area[16]. A minority of patients experience a self-limiting post-ablation syndrome (PAS) characterized by fever, malaise, and chills in the first week[48]. Less than 4% of patients experience serious complications such as bleeding, abscess formation, liver failure, and damage to surrounding structures[49].
Ablation has been considered a curative treatment for early HCC ≤ 3 cm by the NCCN since 2017[39,44]. A study including 120 patients with HCC ≤ 3 cm were randomized into either RFA or resection treatment groups. Results showed insignificant differences in the disease-free and overall survival rates at 1, 2, and 3 years. However, the RFA group exhibited meaningfully better hepatic function a week post-treatment, fewer incidences of postoperative complications, and shorter hospital stays[44]. Another study of RFA efficacy in 218 patients demonstrated complete response for lesions < 2 cm in more than 90% of the cases, with a local recurrence rate of < 1% and no mortality[16]. Studies have demonstrated that ablation of lesions up to 5 cm carries a 5-year overall survival rate of 60% with low rates of local recurrence[12].
RFA and MWA can also be considered in intermediate and advanced stage patients (BCLC B/C) for down-staging to transplantation, with demonstrable success when combined with TACE[50]. Additionally, no significant differences in overall survival were noted between patients who were down-staged via ablation to within Milan criteria before being transplanted, vs transplanted patients who defaulted within the Milan criteria[51]. Ablation can also be considered in patients within the Milan criteria for bridging to transplantation, with RFA leading to a lower waitlist dropout rate than bridging with TACE[52].
Future work should be focused on the role for new or less commonly used ablative modalities in early HCC, including high-intensity focused ultrasound[53], laser ablation[54], and irreversible electroporation[53] (Figure 1).
TARE, also called selective internal radiotherapy (SIRT), is the administration of glass or resin microbeads coated in Yttrium-90 (90Y) via a catheter into the hepatic artery supplying the tumor of interest[55]. This therapy targets tumors by taking advantage of the fact that they are preferentially supplied by hepatic arteries due to neo-angiogenesis, while liver parenchyma is supplied primarily by the portal vein[56]. Unlike for ablation therapy, tumor location in relation to other important structures is less of a concern for catheter-directed therapies; it is more important that the vascular supply to the tumor can be identified and accessed with an intravascular approach.
As a result of the studies reviewed below, TARE has recently been incorporated into the BCLC guidelines as a second-line therapy for early stage HCC. Contraindications include any shunting to the GI tract, excessive lung shunting, complete portal vein occlusion, severe liver dysfunction. Recent efforts to determine both optimal procedural approach and maximum tolerable radiation dose have led to data that suggest a curative role for TARE in early HCC[37,57]. Regarding the optimal procedural approach, there has been a trend towards increasingly selective TARE. In the past, where lobar or even whole liver radiation may have been administered, a segmental approach is now preferred[58]. Regarding radiation dose, recent work suggests targeted doses of 400 Gy or greater are well tolerated and demonstrate complete pathologic necrosis in all patients compared to prior thresholds of 190 Gy (complete response of 100% vs 65% respectively)[59].
Using these techniques, three studies demonstrated the potentially curative role for TARE in early HCC. In 2018, Lewandowski et al[35] published the results of a retrospective study on 70 patients with HCC who were treated with TARE alone. For patients with a single lesion ≤ 5 cm and preserved liver function (Child-Pugh A) who were not candidates for surgery or ablation, overall survival was (comparable to surgical resection) 98%, 66% and 57% at 1-, 3-, 5-year respectively. They also reported median overall survival (OS) of 6.7 years. This cohort with a single lesion ≤ 3 cm had 1-, 3-, 5-year overall survival rates of 100%, 82% and 75% respectively. In addition, this study reported 42.7% (100/234) patients with solitary HCC ≤ 5 cm were successfully down-staged to resection (n = 9) or LT (n = 91) after TARE.
In 2021, Salem et al[37] published the results of the retrospective LEGACY study which included 162 patients with solitary HCC up to 8cm (average size 2.7 cm), preserved liver function (Child-Pugh A), and preserved functional status (ECOG 0-1) who were treated with selective TARE. Patients with prior LRT, LT, resection, or systemic therapy were excluded, as were patients with vascular or extrahepatic disease or significant ascites or encephalopathy. In this study, overall survival at 3-years was 86.6% for patients treated with TARE alone (median dose 410 Gy), and 92.8% for patients who underwent TARE and successfully down-staged to LT (21%; 34/162) or resection (6.8%; 11/162). Local recurrence rate was reported in only 5.6%. Despite the segmental delivery of radiation doses exceeding 400 Gy, there were no cases of REILD, and severe adverse events potentially related to treatment occurred in 5.6% of patients. Of note, analysis of patients who were bridged or down-staged to transplant after treatment with TARE shows similar outcomes to typical liver transplant recipients in terms of overall survival[60,61].
Garin et al[57] further published the DOSISPHERE trial in 2021 on role of TARE. This is a phase 2 multicenter trial comparing lobar TARE using a 120 Gy radiation dose ("standard dosimetry") to delivery of a radiation dose of > 205 Gy to the tumor itself ("personalized dosimetry"). Included patients had local, unresectable advanced disease with at least one lesion ≥ 7 cm. Patients with micro-aggregate albumin (MAA) studies demonstrating poor targeting of the tumor were excluded from the study. Personalized dosimetry was associated with significantly better response rates, defined as partial and complete responders at 3 mo using the European Association for the Study of the Liver (EASL) criteria. Partial and complete responders at 3 mo in the largest lesion were significantly higher in the personalized dosimetry group compared to the standard group: 71% (20/28) vs 36% (10/28). TARE achieved down-staging to resection in 36% (10/28) patients in the personalized dosimetry group and 3.5% (1/28) in the standard dosimetry group, including patients with portal vein tumor thrombus. Overall survival was improved in the personalized dosimetry group (26.6 mo vs 10.7 mo). This trial, while not attempting to demonstrate cure, is significant for its rigorous design, inclusion of patients with larger lesions and more advanced disease (including portal vein thrombus) and remarkable outcomes. Using more selective approaches and higher radiation doses, these studies demonstrates that the best possible outcomes for TARE are yet to come.
While the aforementioned studies used glass beads, future work will clarify appropriate dosing with resin beads. The ongoing DOORwaY90 trial will provide data on overall response and duration of response for resin beads, as well as data on safety, quality of life, bridging and down-staging (NCT04736121) (Table 2).
| LEGACY | DOSISPHERE | |
| Study design | Multi-center single-arm retrospective study | Multicenter randomized control phase ii trial |
| Objective | To assess clinical outcomes of Y-90 glass microsphere treatment in patients with unresectable solitary HCC lesions | To compare clinical outcomes of lobar TARE using 120 Gy (SDA) versus > 205 Gy (PDA) in patients with intermediate/advanced HCC |
| Inclusion criteria | Unresectable solitary lesions (≤ 8 cm); BCLC A or C (ECOG 0-1); Child-Pugh score A | ≥ 1 unresectable lesion ≥ 7 cm; BCLC A, B, or C |
| Exclusion criteria | Patients with vascular or extrahepatic disease, significant ascites, encephalopathy, or prior LRT, LT, resection, or systemic therapy | Patients with micro-aggregate albumin (MAA) studies demonstrating poor tumor targeting |
| Overall survival | At 3 yr, 86.6% for patients treated with TARE alone (median dose 410 Gy) and 92.8% for patients who down-staged via TARE | Overall survival was improved in the personalized dosimetry group (26.6 mo vs 10.7 mo) |
| Downstaging | 21% successfully down-staged to LT; 6.8% to resection. | 36% patients in the PDA group and 3.5% (1/28) in the SDA group down-staged to resection1 |
TACE refers to the delivery of chemotherapy via a catheter within the hepatic artery supplying the tumor. Chemotherapy can be delivered as a liquid solution followed by embolics or as drug-eluting beads. To date, TACE is not generally considered a curative-intent therapy. However, it may be offered to patients with early HCC as second-line therapy for those who are not candidates for surgical approaches or ablation in a process that has been termed stage migration. Typical contraindications include extrahepatic disease, main or lobar portal vein thrombus, and poor liver function (Child-Pugh C). Relative contraindications include elevated total bilirubin (≥ 2-3 mg/dL), as this increases the risk for radio-embolization-induced liver disease (REILD) in TARE and liver failure in TACE.
Novel technical approaches that have demonstrated improved survival are reviewed here. Selective TACE, defined as administration to a segmental artery, and super-selective TACE, defined as administration at the distal portion of a sub-segmental hepatic artery, both improve survival compared to lobar administration[62]. Another described technique is ultra-selective TACE, in which lipoidal is administered to the hepatic artery until opacification of the tumor's portal venous supply is seen[62,63]. In theory, this prevents post-procedural compensatory increase in portal venous supply to the tumor ensuring complete tumor ischemia and multiple studies have demonstrated improved local tumor response using this method[64,65]. While outcomes for selective and super-selective TACE include complete response rates around 40%-50%[62,66] and 5-year overall survival around 20%-35%[67], it is important to consider that TACE has primarily been studied in intermediate and advanced HCC, as opposed to early HCC. In fact, a small prospective study of selective TACE in early HCC (BCLC 0 or A) demonstrated a 3-year survival of 80%[68]. Patients most likely to benefit from TACE include those with fewer lesions and preserved liver function (BCLC A)[63,69]. Additional developments include the use of modified techniques including balloon-occlusion (B-TACE) and microvalve infusion catheters. These have demonstrated improved tumor targeting and greater rates of tumor necrosis but have not yet demonstrated improved clinical outcomes[70,71]. In the case of B-TACE, higher rates of complete response have been observed when compared to conventional TACE[70,72]. Furthermore, there is well-established evidence for the use of TACE in bridging and down-staging to transplantation[73,74].
Barriers to improved outcomes in TACE include lack of technical standards, specific chemotherapeutic agents, and the embolic effect of therapy, which prevents treatment of distal vessels. Thus, future work for curative TACE will require the development of technical standards and improved chemotherapeutics that are both tolerable and effective.
In accordance with recent updates to the BCLC guidelines, TACE and TARE are now both acceptable second-line therapies for early stage HCC. TARE is indicated for single lesions less than 8 cm, whereas TACE is recommended for multifocal disease. Overall survival for TACE vs TARE in HCC has not been directly compared in an RCT. However, a small randomized study demonstrated that TARE led to significantly increased time to progression (> 26 mo vs 6.8 mo) compared to TACE[75]. Other work has suggested an increased time to progression and higher quality of life for TARE compared to TACE[76]. Between TACE and TARE, the 2021 MERITS-LT trial demonstrated no differences in the rate of or time to successful downstaging to LT when either LRT was the initial downstaging treatment[77].
Considerations for choosing one over the other may be guided by patient characteristics. For example, patients with prior biliary instrumentation are higher risk for the development of hepatic abscess after TACE[78]. Despite its name, the ischemic effect of TARE is minimal compared to TACE. As a result, TARE is generally preferred for patients with significant portal vein tumor thrombus, given concerns for excessive ischemia using TACE[79-81].
In solitary HCC lesions up to 7 cm in size, combination therapy with ablation and TACE improves outcomes compared to ablation alone[82]. In 2021, Zhang et al[82] in a RCT of 189 patients, demonstrated superior overall survival for RFA plus TACE compared with RFA alone for early HCC < 7 cm (5-year and 7-year OS of 52% and 36% vs 43% and 19%, respectively). The benefit was particularly pronounced in tumors > 3 cm. This is consistent with prior studies demonstrating the benefit of combination ablation plus embolization in 3-5 cm tumors[83,84].
Given the high rates of recurrence after resection and ablation, the phase 3 STORM trial examined whether the addition of adjuvant sorafenib could reduce the recurrence rate after curative-intent treatment compared to active surveillance, but was unable to demonstrate benefit[85]. Ongoing trials explore the potential for other systemic therapies as adjuvants, including pembrolizumab monotherapy (NCT03867084), nivolumab monotherapy (NCT03383458), atezolizumab plus bevacizumab (NCT04102098), and durvalumab plus bevacizumab (NCT03847428).
The recent addition of TARE as an acceptable stage migration treatment modality for early HCC[86] suggests that future trials on combination treatments for early HCC may increasingly incorporate TARE as a treatment modality.
The treatment of early HCC is evolving, with improved outcomes for transplant, resection, and ablation. Most recently, evidence demonstrates curative outcomes for catheter-directed transarterial therapies for early HCC and suggest that a fourth modality may soon join the list of curative options. Longer follow up periods, technique standardization, and larger randomized controlled trials comparing loco-regional therapies to other curative modalities and defining patients who are most likely to benefit from TARE are needed to confirm these findings.
Zane KE would like to thank Drs. Osman Ahmed, Edward Kim, and Joe Massa for illuminating conversations on transarterial chemoembolization.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta T, India; Limaiem F, Tunisia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1343] [Cited by in F6Publishing: 2564] [Article Influence: 854.7] [Reference Citation Analysis (1)] |
| 2. | Pawlik TM, Vauthey JN, Abdalla EK, Pollock RE, Ellis LM, Curley SA. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg. 2006;141:537-43; discussion 543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Chok KS, Ng KK, Poon RT, Lo CM, Fan ST. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96:81-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 541] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 5. | Kianmanesh R, Regimbeau JM, Belghiti J. Selective approach to major hepatic resection for hepatocellular carcinoma in chronic liver disease. Surg Oncol Clin N Am. 2003;12:51-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, Are C, Brown DB, Chang DT, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Schmidt C, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Zhu AX, Hoffmann KG, Darlow S. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15:563-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 7. | Mazzaferro V, Lencioni R, Majno P. Early hepatocellular carcinoma on the procrustean bed of ablation, resection, and transplantation. Semin Liver Dis. 2014;34:415-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182-8; discussion 188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 647] [Cited by in F6Publishing: 664] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 10. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4333] [Cited by in F6Publishing: 4404] [Article Influence: 231.8] [Reference Citation Analysis (0)] |
| 11. | Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M, Blanc JF, Johnson P, Kudo M, Roberts LR, Sherman M. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 12. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-77; quiz 578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 13. | Brunello F, Cantamessa A, Gaia S, Carucci P, Rolle E, Castiglione A, Ciccone G, Rizzetto M. Radiofrequency ablation: technical and clinical long-term outcomes for single hepatocellular carcinoma up to 30 mm. Eur J Gastroenterol Hepatol. 2013;25:842-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Vincenzi B, Di Maio M, Silletta M, D'Onofrio L, Spoto C, Piccirillo MC, Daniele G, Comito F, Maci E, Bronte G, Russo A, Santini D, Perrone F, Tonini G. Prognostic Relevance of Objective Response According to EASL Criteria and mRECIST Criteria in Hepatocellular Carcinoma Patients Treated with Loco-Regional Therapies: A Literature-Based Meta-Analysis. PLoS One. 2015;10:e0133488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 98] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 15. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 274] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 16. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 774] [Cited by in F6Publishing: 783] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 17. | Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015;21:1142-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 18. | Xing M, Kim HS. Independent prognostic factors for posttransplant survival in hepatocellular carcinoma patients undergoing liver transplantation. Cancer Med. 2017;6:26-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 1000] [Article Influence: 200.0] [Reference Citation Analysis (1)] |
| 20. | Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 21. | Doyle MB, Vachharajani N, Maynard E, Shenoy S, Anderson C, Wellen JR, Lowell JA, Chapman WC. Liver transplantation for hepatocellular carcinoma: long-term results suggest excellent outcomes. J Am Coll Surg. 2012;215:19-28; discussion 28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Filgueira NA. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J Hepatol. 2019;11:261-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 13.8] [Reference Citation Analysis (3)] |
| 23. | Alim A, Erdogan Y, Dayangac M, Yuzer Y, Tokat Y, Oezcelik A. Living Donor Liver Transplantation: The Optimal Curative Treatment for Hepatocellular Carcinoma Even Beyond Milan Criteria. Cancer Control. 2021;28:10732748211011960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Shin S, Kim TS, Lee JW, Ahn KS, Kim YH, Kang KJ. Is the anatomical resection necessary for single hepatocellular carcinoma smaller than 3 cm? Ann Hepatobiliary Pancreat Surg. 2018;22:326-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 26. | Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4:7252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Dou J, Cheng Z, Han Z, Liu F, Wang Z, Yu X, Yu J, Liang P. Microwave ablation vs. surgical resection for treatment naïve hepatocellular carcinoma within the Milan criteria: a follow-up of at least 5 years. Cancer Biol Med. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Lee DH, Kim JW, Lee JM, Kim JM, Lee MW, Rhim H, Hur YH, Suh KS. Laparoscopic Liver Resection versus Percutaneous Radiofrequency Ablation for Small Single Nodular Hepatocellular Carcinoma: Comparison of Treatment Outcomes. Liver Cancer. 2021;10:25-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 29. | Vitali GC, Laurent A, Terraz S, Majno P, Buchs NC, Rubbia-Brandt L, Luciani A, Calderaro J, Morel P, Azoulay D, Toso C. Minimally invasive surgery versus percutaneous radio frequency ablation for the treatment of single small (≤3 cm) hepatocellular carcinoma: a case-control study. Surg Endosc. 2016;30:2301-2307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 31. | Hao Y, Numata K, Ishii T, Fukuda H, Maeda S, Nakano M, Tanaka K. Rate of local tumor progression following radiofrequency ablation of pathologically early hepatocellular carcinoma. World J Gastroenterol. 2017;23:3111-3121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Lee MW, Kang D, Lim HK, Cho J, Sinn DH, Kang TW, Song KD, Rhim H, Cha DI, Lu DSK. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma < 3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020;30:2391-2400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 33. | Ueno M, Takabatake H, Itasaka S, Kayahara T, Morimoto Y, Yamamoto H, Mizuno M. Stereotactic body radiation therapy versus radiofrequency ablation for single small hepatocellular carcinoma: a propensity-score matching analysis of their impact on liver function and clinical outcomes. J Gastrointest Oncol. 2021;12:2334-2344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, Kulik L, Ganger D, Desai K, Thornburg B, Mouli S, Hickey R, Caicedo JC, Abecassis M, Riaz A, Salem R. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018;287:1050-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 35. | Biederman DM, Titano JJ, Bishay VL, Durrani RJ, Dayan E, Tabori N, Patel RS, Nowakowski FS, Fischman AM, Kim E. Radiation Segmentectomy versus TACE Combined with Microwave Ablation for Unresectable Solitary Hepatocellular Carcinoma Up to 3 cm: A Propensity Score Matching Study. Radiology. 2017;283:895-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, Fowers K, Lewandowski R, Padia SA. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology. 2021;74:2342-2352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 37. | Sun Q, Shi J, Ren C, Du Z, Shu G, Wang Y. Survival analysis following microwave ablation or surgical resection in patients with hepatocellular carcinoma conforming to the Milan criteria. Oncol Lett. 2020;19:4066-4076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 38. | Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, Yuen J, Poon RTP, Fan ST, Lo CM. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775-1784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 39. | Huang Z, Guo Z, Ni J, Zuo M, Zhang T, Ma R, An C, Huang J. Four types of tumor progression after microwave ablation of single hepatocellular carcinoma of ≤5 cm: incidence, risk factors and clinical significance. Int J Hyperthermia. 2021;38:1164-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aubé C, Digklia A, Pache I, Deltenre P, Knebel JF, Denys A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 41. | Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, Mulcahy M, Baker T, Abecassis M, Sato KT, Caicedo JC, Fryer J, Hickey R, Hohlastos E, Lewandowski RJ, Salem R. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 42. | Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21:S187-S191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 43. | Fang Y, Chen W, Liang X, Li D, Lou H, Chen R, Wang K, Pan H. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 580] [Cited by in F6Publishing: 591] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 45. | National Comprehensive Cancer Network. Hepatobiliary Cancers (version 5.2021).Available from: https://www.nccn.org/home/news/ebulletin-newsletter-archive#. [Cited in This Article: ] |
| 46. | Roayaie S, Jibara G, Taouli B, Schwartz M. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol. 2013;20:3754-3760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Dodd GD, Napier D, Schoolfield JD, Hubbard L. Percutaneous radiofrequency ablation of hepatic tumors: postablation syndrome. AJR Am J Roentgenol. 2005;185:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584-2596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | English K, Brodin NP, Shankar V, Zhu S, Ohri N, Golowa YS, Cynamon J, Bellemare S, Kaubisch A, Kinkhabwala M, Kalnicki S, Garg MK, Guha C, Kabarriti R. Association of Addition of Ablative Therapy Following Transarterial Chemoembolization With Survival Rates in Patients With Hepatocellular Carcinoma. JAMA Netw Open. 2020;3:e2023942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, Nalesnik M, Geller DA, Steel JL, Gamblin TC. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15:3169-3177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Huo TI, Huang YH, Su CW, Lin HC, Chiang JH, Chiou YY, Huo SC, Lee PC, Lee SD. Validation of the HCC-MELD for dropout probability in patients with small hepatocellular carcinoma undergoing locoregional therapy. Clin Transplant. 2008;22:469-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Zavaglia C, Mancuso A, Foschi A, Rampoldi A. High-intensity focused ultrasound (HIFU) for the treatment of hepatocellular carcinoma: is it time to abandon standard ablative percutaneous treatments? Hepatobiliary Surg Nutr. 2013;2:184-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 53. | Di Costanzo GG, Francica G, Pacella CM. Laser ablation for small hepatocellular carcinoma: State of the art and future perspectives. World J Hepatol. 2014;6:704-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Zimmerman A, Grand D, Charpentier KP. Irreversible electroporation of hepatocellular carcinoma: patient selection and perspectives. J Hepatocell Carcinoma. 2017;4:49-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 56. | Sutphin PD, Lamus D, Kalva SP, Li J, Corbin IR. Interventional Radiologic Therapies for Hepatocellular Carcinoma: From Where We Began to Where We Are Going. 2019 Aug 6. In: Hepatocellular Carcinoma: Translational Precision Medicine Approaches [Internet]. Cham (CH): Humana Press; 2019–. [PubMed] [Cited in This Article: ] |
| 57. | Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, Assenat E, Tacher V, Robert C, Terroir-Cassou-Mounat M, Mariano-Goulart D, Amaddeo G, Palard X, Hollebecque A, Kafrouni M, Regnault H, Boudjema K, Grimaldi S, Fourcade M, Kobeiter H, Vibert E, Le Sourd S, Piron L, Sommacale D, Laffont S, Campillo-Gimenez B, Rolland Y; DOSISPHERE-01 Study Group. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 271] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 58. | Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, Sato KT, Baker T, Kulik L, Gupta R, Abecassis M, Benson AB 3rd, Omary R, Millender L, Kennedy A, Salem R. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Gabr A, Riaz A, Johnson GE, Kim E, Padia S, Lewandowski RJ, Salem R. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging. 2021;48:580-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 415] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 61. | Gabr A, Kulik L, Mouli S, Riaz A, Ali R, Desai K, Mora RA, Ganger D, Maddur H, Flamm S, Boike J, Moore C, Thornburg B, Alasadi A, Baker T, Borja-Cacho D, Katariya N, Ladner DP, Caicedo JC, Lewandowski RJ, Salem R. Liver Transplantation Following Yttrium-90 Radioembolization: 15-Year Experience in 207-Patient Cohort. Hepatology. 2021;73:998-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 62. | Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, Ravaioli M, D'Errico-Grigioni A, Pinna AD, Bolondi L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 63. | Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, Miyayama S, Cheng AL. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020;9:245-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 64. | Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, Takeda T, Yoneda N, Notsumata K, Toya D, Tanaka N, Mitsui T. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18:365-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 65. | Bannangkoon K, Hongsakul K, Tubtawee T, Piratvisuth T. Safety margin of embolized area can reduce local recurrence of hepatocellular carcinoma after superselective transarterial chemoembolization. Clin Mol Hepatol. 2019;25:74-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Albrecht KC, Aschenbach R, Diamantis I, Eckardt N, Teichgräber U. Response rate and safety in patients with hepatocellular carcinoma treated with transarterial chemoembolization using 40-µm doxorubicin-eluting microspheres. J Cancer Res Clin Oncol. 2021;147:23-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, Marinis A, Kelekis A, Alexopoulou E, Chatziioannou A, Chatzimichael K, Dourakis S, Kelekis N, Rizos S, Kelekis D. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35:1119-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 68. | Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, Cicorelli A, Tumino E, Federici G, Cioni R, Metrangolo S, Bertoni M, Bresci G, Parisi G, Altomare E, Capria A, Bartolozzi C. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Takayasu K, Arii S, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y, Kokudo N, Makuuchi M. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56:886-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 70. | Irie T, Kuramochi M, Kamoshida T, Takahashi N. Selective balloon-occluded transarterial chemoembolization for patients with one or two hepatocellular carcinoma nodules: Retrospective comparison with conventional super-selective TACE. Hepatol Res. 2016;46:209-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Titano JJ, Fischman AM, Cherian A, Tully M, Stein LL, Jacobs L, Rubin RA, Bosley M, Citron S, Joelson DW, Shrestha R, Arepally A. End-hole Versus Microvalve Infusion Catheters in Patients Undergoing Drug-Eluting Microspheres-TACE for Solitary Hepatocellular Carcinoma Tumors: A Retrospective Analysis. Cardiovasc Intervent Radiol. 2019;42:560-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Ogawa M, Takayasu K, Hirayama M, Miura T, Shiozawa K, Abe M, Matsumoto N, Nakagawara H, Ohshiro S, Yamamoto T, Tanaka N, Moriyama M, Mutou H, Yamamoto Y, Irie T. Efficacy of a microballoon catheter in transarterial chemoembolization of hepatocellular carcinoma using miriplatin, a lipophilic anticancer drug: Short-term results. Hepatol Res. 2016;46:E60-E69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D'Errico Grigioni A, Panzini I, Morelli C, Bernardi M, Bolondi L, Pinna AD. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547-2557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 74. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 75. | Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155-1163.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 426] [Article Influence: 53.3] [Reference Citation Analysis (30)] |
| 76. | Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv Ther. 2016;33:699-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 77. | Mehta N, Frenette C, Tabrizian P, Hoteit M, Guy J, Parikh N, Ghaziani TT, Dhanasekaran R, Dodge JL, Natarajan B, Holzner ML, Frankul L, Chan W, Fobar A, Florman S, Yao FY. Downstaging Outcomes for Hepatocellular Carcinoma: Results From the Multicenter Evaluation of Reduction in Tumor Size before Liver Transplantation (MERITS-LT) Consortium. Gastroenterology. 2021;161:1502-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 78. | Lv WF, Lu D, He YS, Xiao JK, Zhou CZ, Cheng DL. Liver Abscess Formation Following Transarterial Chemoembolization: Clinical Features, Risk Factors, Bacteria Spectrum, and Percutaneous Catheter Drainage. Medicine (Baltimore). 2016;95:e3503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Fidelman N, Kerlan RK Jr. Transarterial Chemoembolization and (90)Y Radioembolization for Hepatocellular Carcinoma: Review of Current Applications Beyond Intermediate-Stage Disease. AJR Am J Roentgenol. 2015;205:742-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Rahman SI, Nunez-Herrero L, Berkes JL. Position 2: Transarterial Radioembolization Should Be the Primary Locoregional Therapy for Unresectable Hepatocellular Carcinoma. Clin Liver Dis (Hoboken). 2020;15:74-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Zane KE, Makary MS. Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Zhang YJ, Chen MS, Chen Y, Lau WY, Peng Z. Long-term Outcomes of Transcatheter Arterial Chemoembolization Combined With Radiofrequency Ablation as an Initial Treatment for Early-Stage Hepatocellular Carcinoma. JAMA Netw Open. 2021;4:e2126992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 83. | Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK, Sung KB, Kim PN. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012;81:e189-e193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 84. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 85. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 664] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 86. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 1460] [Article Influence: 730.0] [Reference Citation Analysis (41)] |