Copyright
©The Author(s) 2020.
World J Hepatol. Sep 27, 2020; 12(9): 596-618
Published online Sep 27, 2020. doi: 10.4254/wjh.v12.i9.596
Published online Sep 27, 2020. doi: 10.4254/wjh.v12.i9.596
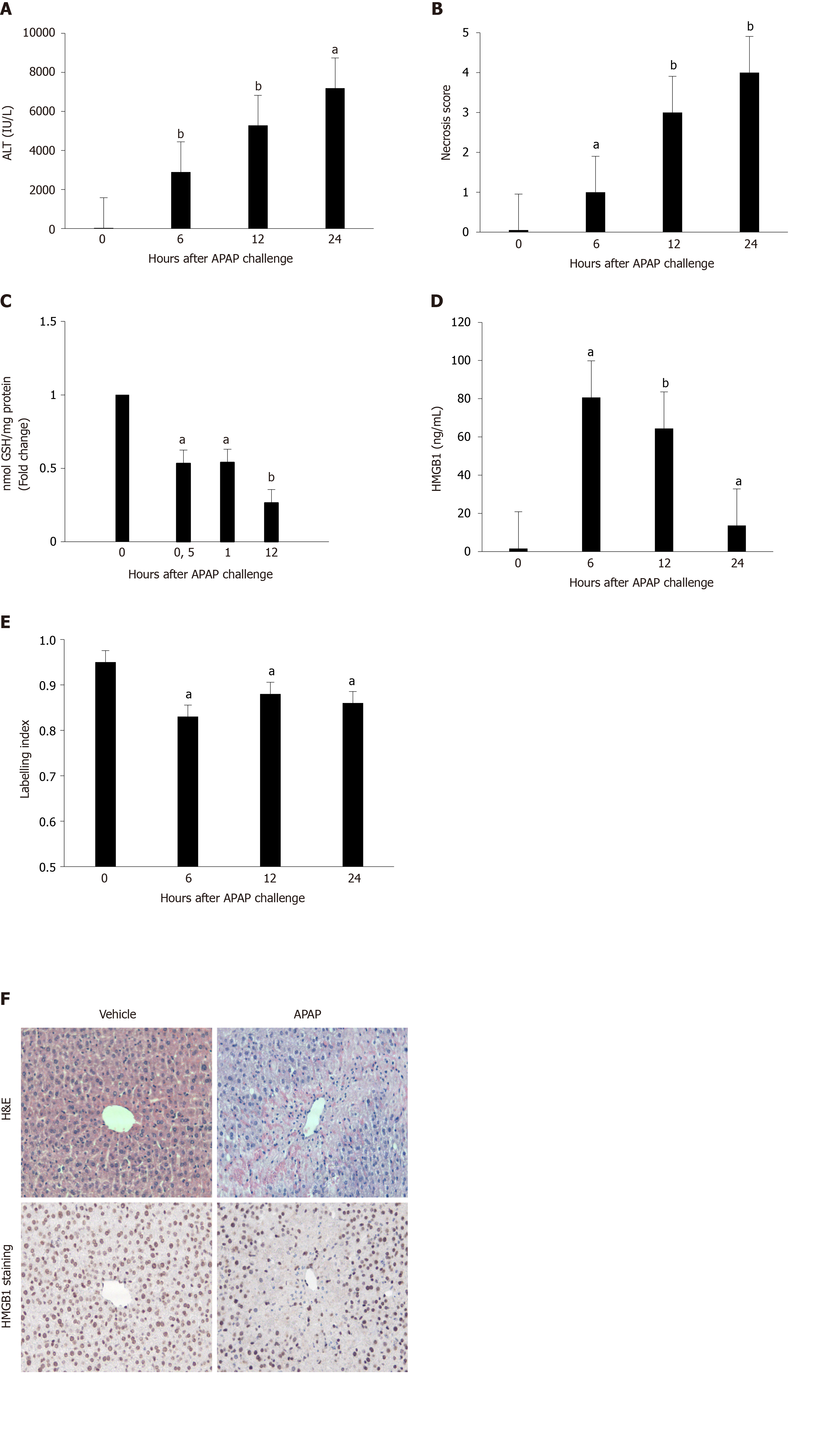
Figure 1 Murine model of acetaminophen-induced liver injury: Hepatotoxicity assessment.
A: Alanine aminotransferase (ALT) levels were measured in sera of vehicle-treated mice (0 h) and in sera of mice sacrificed 2, 6, 12 or 24 h after acetaminophen [APAP; 500 mg/kg] administration (5 mice in each group); B: Liver necrosis was scored in the same groups of mice; C: Hepatic glutathione (GSH) levels were measured at 30 min, 1 and 12 h after APAP challenge. The enzyme concentration obtained is expressed as nanomoles of enzyme per milligram of protein using bovine serum as a standard; D: High mobility group box 1 (HMGB1) levels were measured in the same groups of mice; E: Quantification of nuclear expression of HMGB1 in the same groups of mice; F: Representative hematoxylin and eosin (magnification × 200) and HMGB1-stained images (magnification × 200) of murine liver 24 h after vehicle or APAP challenge. Results are expressed as mean ± standard error. aP < 0.05, bP < 0.01 vs 0. Experiments were reproduced three times. H&E: Hematoxylin and eosin.
- Citation: Minsart C, Rorive S, Lemmers A, Quertinmont E, Gustot T. N-acetylcysteine and glycyrrhizin combination: Benefit outcome in a murine model of acetaminophen-induced liver failure. World J Hepatol 2020; 12(9): 596-618
- URL: https://www.wjgnet.com/1948-5182/full/v12/i9/596.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i9.596