Copyright
©The Author(s) 2024.
World J Hepatol. Apr 27, 2024; 16(4): 494-505
Published online Apr 27, 2024. doi: 10.4254/wjh.v16.i4.494
Published online Apr 27, 2024. doi: 10.4254/wjh.v16.i4.494
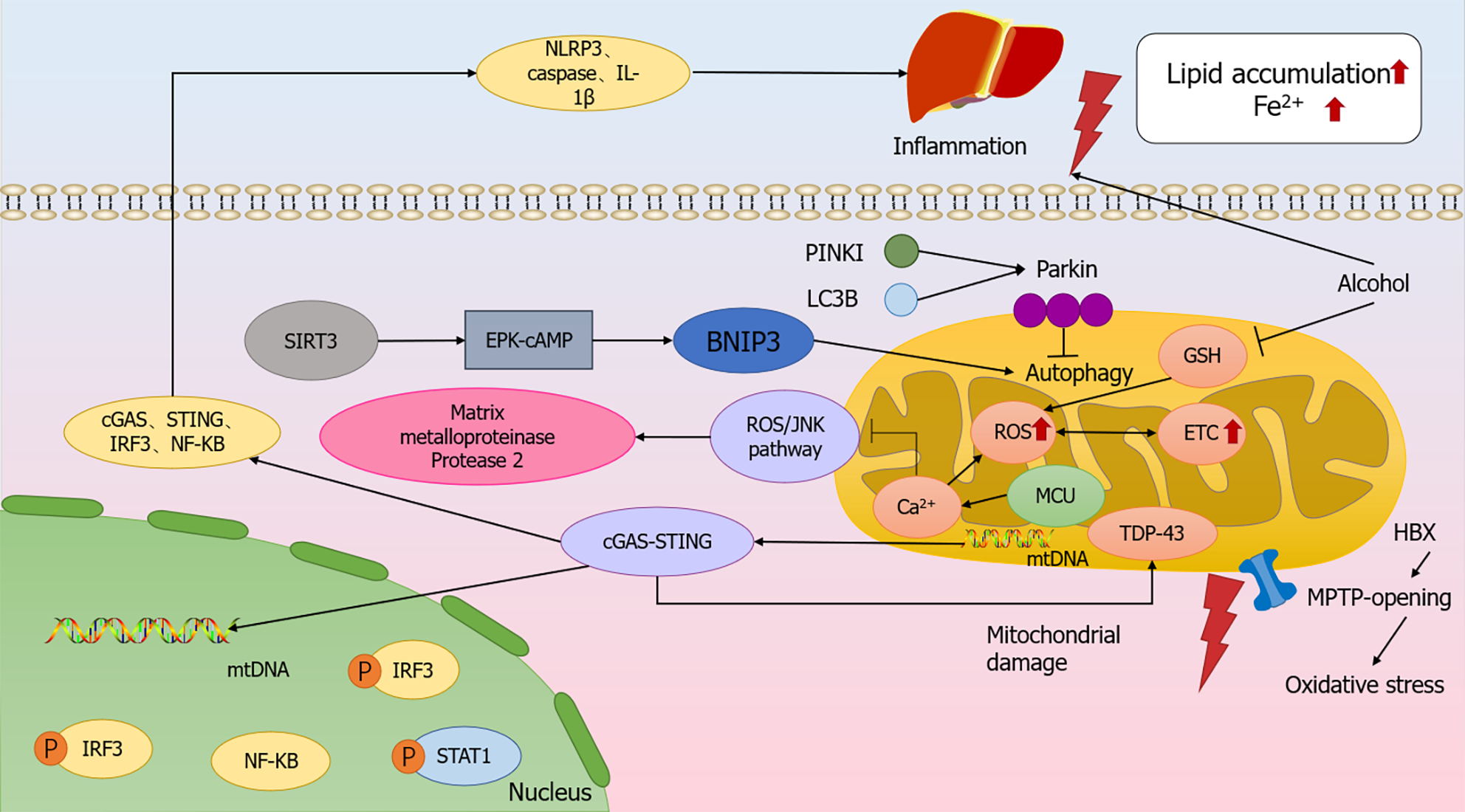
Figure 1 Molecular mechanism of mitochondrial dysfunction modulating the relationship between chronic liver disease.
Increasing electron transport chain (ETC) leads to excess reactive oxygen species (ROS), causing corresponding metabolic changes and ultimately ETC dysfunction. Alcohol metabolism, which decreases GSH levels in mitochondria, increases ROS production and causes iron death. Interfering with autophagy by silencing Parkin led to enhanced apoptotic signaling, while SIRT3 increased BNIP3 expression in hepatocytes via the ERK-cAMP pathway, promoting BNIP3-mediated mitochondrial autophagy. Mitochondrial injury can lead to mtDNA leakage, which triggers an inflammatory response through the cGAS-String pathway. cGAS-STRING signaling pathway key molecules, cGAS, STING, and IRF3 expression levels, as well as the downstream NF-κB nuclear translocation, were significantly increased, and at the same time, it caused the accumulation of TDP-43 in the mitochondria of hepatocytes, which induced mitochondrial damage. Inflammatory response significantly increased the expression levels of NLRP3, caspasel and IL-1β in the liver, resulting in iron accumulation and lipid accumulation. ETC: Electron transport chain; ROS: Reactive oxygen species.
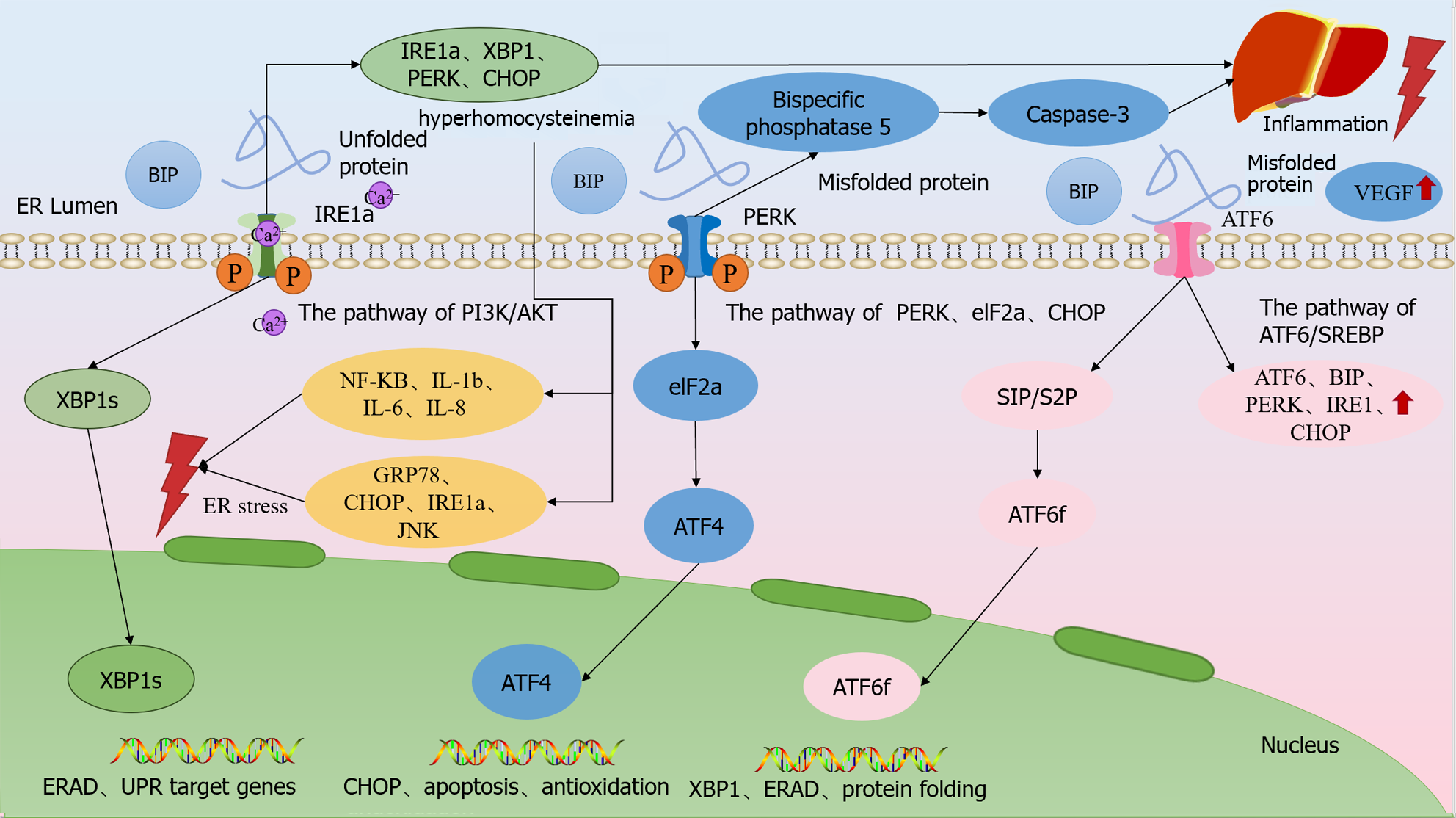
Figure 2 Endoplasmic reticulum dysfunction modulates chronic liver disease.
Upon occurrence of endoplasmic reticulum stress (ERS), BiP dissociates from the three ER transmembrane proteins and binds to unfolded proteins with high affinity, and the dissociated transmembrane proteins shift to an active state and activate downstream signaling. Upon dissociation from BiP upon ERS, PERK endoplasmic reticulum stress increases the expression of bispecific phosphatase 5 in hepatocytes through the PERK/eIF2α/CHOP pathway as a means of raising the intracellular activated caspase-3 levels, which ultimately induces hepatocyte death. ATF6 is cleaved upon dissociation from BiP during ERS onset, and cleaved ATF6α activates the transcription of XBP1u.IRE1α is activated and translocates to the cell membrane via the PI3K/AKT pathway, leading to extracellular Ca2+ inward flow, which disrupts the intracellular calcium homeostasis and triggers ERS, so that IRE1α, XBP1, PERK and CHOP upregulation, which ultimately leads to hepatic stellate cell activation and proliferation and promotes liver fibrosis. ER: Endoplasmic reticulum; VEGF: Vascular endothelial growth factor.
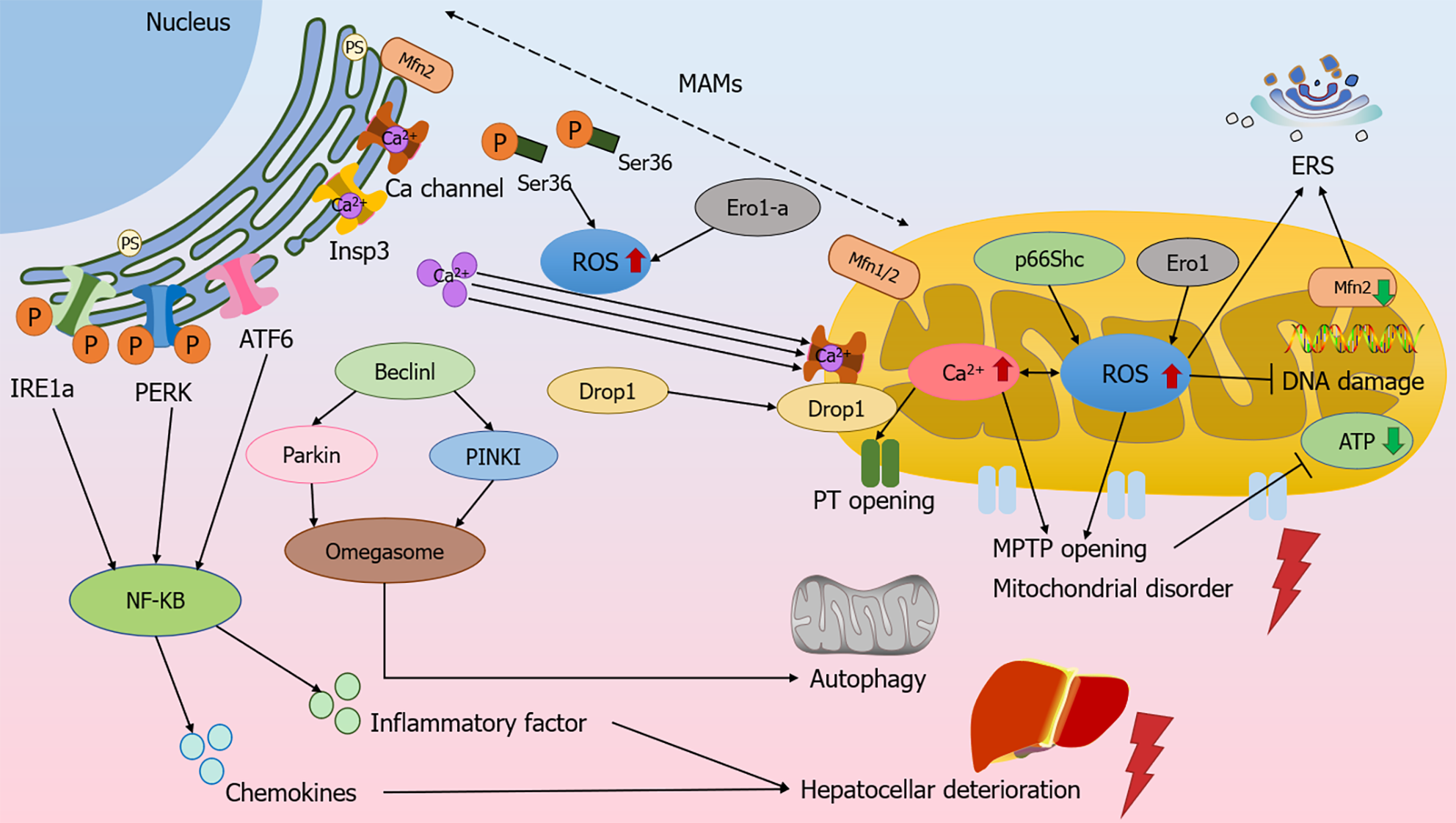
Figure 3 Mitochondrial and endoplasmic reticulum-associated signaling regulates the molecular mechanism of chronic liver disease.
Reactive oxygen species (ROS) in the endoplasmic reticulum (ER) initiates Ca2+ efflux from Insp3 and Ca ion channels, and the Ser36 site is dephosphorylated and can be transferred to mitochondria-associated ER membranes (MAMs) to mediate ROS production. ROS promotes Ca2+ in the ER to flow to the mitochondria through the MAMs and increases mitochondrial ROS production, and conversely, the increase in ROS affects Ca2+ and initiates the opening of permeability transition pores, and the swelling of the mitochondria causes the rupture of the outer membrane, which can lead to oxidative damage to DNA and cause adenosine triphosphate depletion. Beclinl and PINK1/Parkin mediate mitochondrial autophagy, and together with PINKI, promote an increase in MAMs and omegasome formation. Excessive accumulation of ROS leads to endoplasmic reticulum stress, activation of the ATF6, IRE1, and PERK, the three unfolded protein response pathways, and the up-regulation of NF-κB activity, promoting the secretion of hepatocyte inflammatory factors and chemokines, leading to functional deterioration of hepatocytes. MAMs: Mitochondria-associated ER membranes; ERS: Endoplasmic reticulum stress; ATP: Adenosine triphosphate.
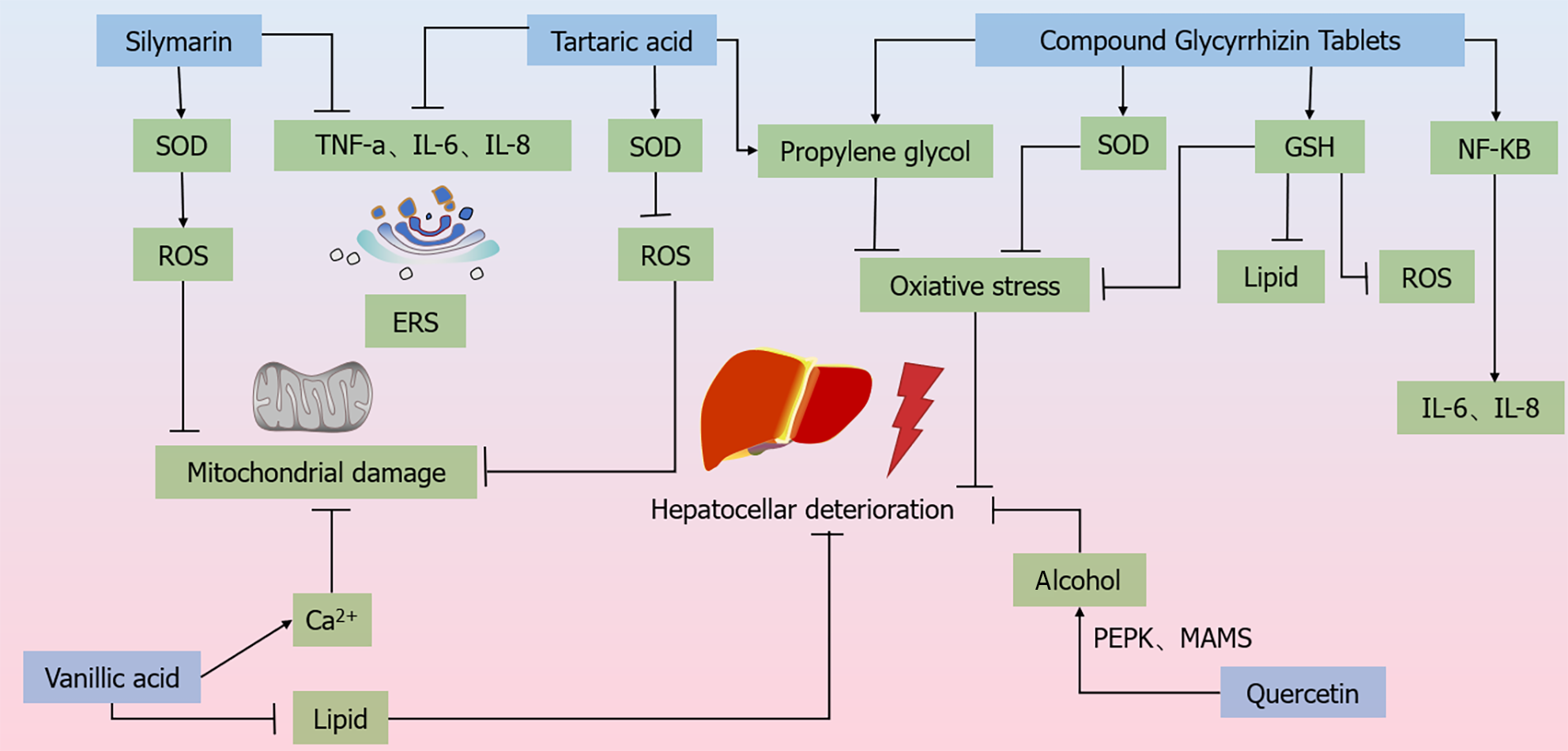
Figure 4 Molecular mechanism of Chinese medicines regulating the treatment of chronic liver disease.
Silymarin increases the expression of superoxide dismutase (SOD) to reduce the levels of reactive oxygen species (ROS) and TNF-α, IL-6, IL-8, etc. to stabilize the endoplasmic reticulum (ER). Rhein is capable of anti-lipid peroxidation and scavenging of ROS, and inhibits the activities of TNF-a and IL-1β to stabilize the ER. vA significantly reduces malondialdehyde and elevates the levels of SOD to reduce ROS in mitochondria. vA has the ability to reduce lipid accumulation in hepatocytes and protect ER and Ca2+ homeostasis. quercetin inhibits alcohol and Ca2+ homeostasis through the regulation of PERK-MAMs pathway. VA has the ability to reduce intracellular lipid accumulation in hepatocytes and effectively protect ER and Ca2+ homeostasis. quercetin inhibits alcohol-induced iron death in hepatocytes and alleviates alcoholic liver injury through the regulation of the PERK-MAMs pathway. Composite glycyrrhizin tablets inhibit NF-κB pro-inflammatory signaling and IL-6 and IL-1β activities by inhibiting NF-κB pro-inflammatory signaling and IL-6 and IL-1β activities. transduction and IL-6 and IL-8 expression to inhibit hepatic injury, and also by promoting SOD and GSH expression as well as inhibiting malondialdehyde synthesis, induced hepatocellular injury. GSH passages inhibit ROS generation and attenuate hepatic injury caused by oxidative stress. ROS: Reactive oxygen species; ERS: Endoplasmic reticulum stress; SOD: Superoxide dismutase.
- Citation: Zheng Y, Zheng YH, Wang JH, Zhao TJ, Wang L, Liang TJ. Progress of mitochondrial and endoplasmic reticulum-associated signaling and its regulation of chronic liver disease by Chinese medicine. World J Hepatol 2024; 16(4): 494-505
- URL: https://www.wjgnet.com/1948-5182/full/v16/i4/494.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i4.494