Copyright
©The Author(s) 2015.
World J Stem Cells. Apr 26, 2015; 7(3): 596-604
Published online Apr 26, 2015. doi: 10.4252/wjsc.v7.i3.596
Published online Apr 26, 2015. doi: 10.4252/wjsc.v7.i3.596
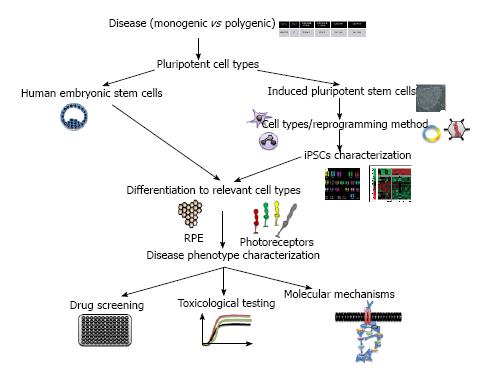
Figure 1 Schematic diagram summarizing the issues to consider when modeling retinal diseases with human pluripotent stem cells.
Choice of disease (monogenic vs polygenic); Type of pluripotent stem cells (hESCs and/or iPSCs); for iPSCs: selection of cell type to reprogram (fibroblasts, keratinocytes, blood cells), selection of reprogramming method (retrovirus mediated, non-integrative); choice of quality control for iPSCs characterization (genetic analysis, pluripotency, teratoma assay); differentiation to relevant cell types (photoreceptors, RPE); disease phenotype characterization (molecular characterization, expression studies, genetic rescue); applications (drug screening, toxicological testing, new molecular mechanisms). hESCs: Human embryonic stem cells; iPSCs: Induced pluripotent stem cells; RPE: Retinal pigment epithelium.
- Citation: Ben M’Barek K, Regent F, Monville C. Use of human pluripotent stem cells to study and treat retinopathies. World J Stem Cells 2015; 7(3): 596-604
- URL: https://www.wjgnet.com/1948-0210/full/v7/i3/596.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i3.596