Copyright
©2014 Baishideng Publishing Group Co.
World J Stem Cells. Jan 26, 2014; 6(1): 1-10
Published online Jan 26, 2014. doi: 10.4252/wjsc.v6.i1.1
Published online Jan 26, 2014. doi: 10.4252/wjsc.v6.i1.1
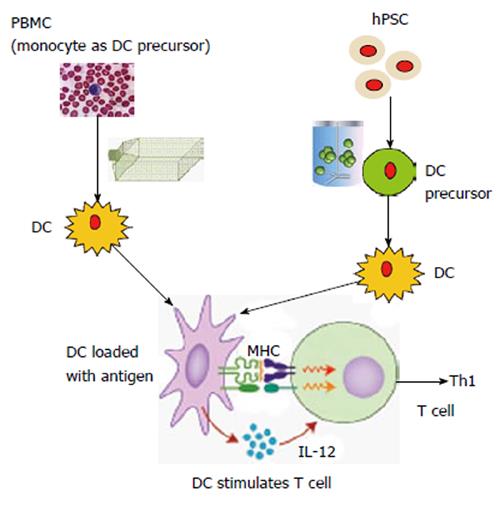
Figure 1 Schematic comparison between conventional dendritic cell vaccine production and human pluripotent stem cell-based dendritic cell C vaccine production.
Conventional dendritic cell (DC) vaccine production is usually from peripheral blood mononuclear cells (PBMCs) in patients. T-flasks, Cell Factories or bags are used for DC maturation. From human pluripotent stem cells (hPSCs), DC precursors (similar to monocytes isolated from PBMCs) can be generated in large scale in bioreactors and then mature into DCs. In theory, this approach can generate an unlimited number of DCs. To stimulate T cell response, the activated DCs can release cytokines such as interleukin (IL)-12 to trigger T helper type 1 (Th1) immune response. DCs are also able to capture and process antigens, converting proteins to peptides that are presented on major histocompatibility complex (MHC) molecules and recognized by T cells. The induced Th1 immune response can target on the cancer cells, which express tumor-specific antigens.
- Citation: Li Y, Liu M, Yang ST. Dendritic cells derived from pluripotent stem cells: Potential of large scale production. World J Stem Cells 2014; 6(1): 1-10
- URL: https://www.wjgnet.com/1948-0210/full/v6/i1/1.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i1.1