Published online Jan 26, 2012. doi: 10.4252/wjsc.v4.i1.1
Revised: November 6, 2011
Accepted: November 15, 2011
Published online: January 26, 2012
AIM: To improve the isolation and expansion of human marrow-derived mesenchymal stem cells (MSCs) based on rat samples.
METHODS: Based on the fact that rat MSCs are relatively easy to obtain from a small aspirate, bone marrow-derived MSCs from rat were cultured and characterized to set up the different protocols used in this study. Then, accordingly, almost the same protocols were performed on human healthy bone marrow samples, after obtaining approval of the ethics committee and gaining informed consent. We used different protocols and culture conditions, including the type of basal media and the culture composition. The MSCs were characterized by immunophenotyping and differentiation.
RESULTS: There was no difference in morphology and proliferation capacity between different culture media at the first passage. During the 5-7th passages, the cells gradually lost their morphology and proliferation potential on Dulbecco’s modified Eagle’s medium (DMEM) high glucose and α modified Eagle’s medium. Although the cells expanded rapidly for up to 10 passages on DMEM low glucose containing 10% to 15% fetal calf serum (FCS), their proliferation was arrested without change in morphology and differentiation capacity at the third passage on 5% FCS. Flow cytometric analysis and functional tests confirmed that more than 90% of marrow cells which were isolated and expanded by our selective protocols were MSCs.
CONCLUSION: We improved the isolation and expansion of human bone marrow derived MSCs, based on rat sample experiments, for further experimental and clinical use.
- Citation: Ayatollahi M, Salmani MK, Geramizadeh B, Tabei SZ, Soleimani M, Sanati MH. Conditions to improve expansion of human mesenchymal stem cells based on rat samples. World J Stem Cells 2012; 4(1): 1-8
- URL: https://www.wjgnet.com/1948-0210/full/v4/i1/1.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v4.i1.1
Mesenchymal stem cells (MSCs) were first reported by Fridenstein et al[1] in 1976. The interest in MSCs rapidly grew with expanding knowledge about their exceptional characteristics and usefulness in the clinic[2-5]. The main source of MSCs is bone marrow. They constitute, however, only a small percentage of the total number of bone marrow cells. In mice, the frequency was estimated to be one for 11 300-27 000 nucleated cells[6] and in humans there is one MSC per 34 000 nucleated cells[7]. Although these cells are present in very low numbers in bone marrow, they are relatively easy to proliferate and expand in proper culture conditions[8]. However, human MSCs are known to constitute a heterogeneous population of cells with different functions. Therefore, their properties depend on environmental conditions[8,9].
Previous studies have shown that human MSCs which have been expanded in vitro tend to lose their proliferative potential, homing capacity, in vivo bone forming efficiency aging and differentiation into other lineages[10-12]. Moreover, the maintenance of MSCs in the undifferentiated phenotype depend on efficient methods of isolation and optimal conditions for subsequent culture supplements[13,14], as well as starting and passaging cell-plate density[15]. Considering the lack of a uniform approach for rapid expansion of human MSCs among laboratories, establishing an optimal cell culture system for in vitro expansion of MSCs is of critical importance.
On the basis of the fact that rat MSCs are relatively easy to obtain from a small aspirate and because rat has also become an often-used model species for human disease, the establishment of a culture system for rat MSCs is beneficial as a prototype for human MSC expansion and differentiation.
Our project followed two main goals: (1) To improve isolation and culture of human mesenchymal cells based on the rat sample; and (2) To analyze the morphology, immunophenotype and differentiation potential of human and rat MSCs after developing a selective culture condition system.
Rat MSCs were isolated from male Sprague Dawley rats (4-6 wk old) and cultured, as will be described later[16]. Prior to the study, all protocols were approved by our institution’s animal welfare regulatory committee. The nucleated cells were seeded directly at 9 × 105 cells/cm2 on collagen-coated culture plates (Nunc) instead of using Ficoll gradient. The plates were divided into five groups. Rat bone marrow cells were cultured in basic media: (1) α modified Eagle’s medium (α-MEM) (Gibco) containing 10% fetal calf serum (FCS) (Gibco); (2) Dulbecco’s modified Eagle’s medium (DMEM) high glucose (4500 mg/L) (Gibco) containing 10% FCS; (3) DMEM low glucose (1000 mg/L) containing 5% FCS; (4) DMEM low glucose containing 10% FCS; and (5) DMEM low glucose containing 15% FCS. There were three plates for each group. The basic media contained 1% penicillin (Invitrogen, Merelbeke, Belgium), 1% streptomycin (Invitrogen, Merelbeke, Belgium) and 2 mmol/L glutamine (Invitrogen, Merelbeke, Belgium).
After 3-4 d, the non-adherent rat cells were removed and the media were changed every 3 d. In order to expand the MSCs, the adhered monolayer was detached with trypsin EDTA (Invitrogen, Merelbeke, Belgium) for 5 min at 37 °C, after 7-9 d for the first passage and every 3-4 d for successive passages in all rat samples. During in vitro passaging, the cells were expanded for several passages until they no longer reached confluence.
Human MSCs were obtained from 5 mL iliac crest aspirates of normal donors who had undergone bone marrow collection for a related patient (age range of 19-49 years) after being approved by the Ethics Committee of Shiraz University of Medical Sciences. Written informed consent was obtained, allowing analysis of the clinical data and tests mentioned in this study. Each sample of the aspirate was diluted 1:1 with DMEM low glucose and layered over about 5 mL of Ficoll (Lymphoprep; Oslo, Norway). The isolation method was performed according to the two previously reported methods[17,18] and our selective method which has been mentioned briefly. After centrifugation at 2000 rpm for 30 min, the mononuclear cell layer was removed from the interface. The cells were suspended in DMEM, centrifuged at 1200 rpm for 15 min and then resuspended in basal DMEM low glucose containing 10% fetal calf serum, 1% penicillin, 1% streptomycin and 2 mmol/L glutamine. The cells were seeded at a density of 80.000/cm2 in 25 cm2 T-flasks and maintained at 37 °C with an atmosphere of 5% CO2. Culture medium was changed every three days until the samples were harvested.
At each passage, the cells were counted and analyzed for viability by trypan blue staining analysis and at the third passage immunophenotype analysis by cytoflurimetric assay. The functional potential of differentiation into osteocyte and adipocyte was also achieved in response to specific culture conditions.
At the third passage, the cells were detached from the culture flasks with trypsin EDTA and counted. The identification of adherent cells was performed by flow cytometric analysis according to the previously reported method[19]. Subsequently, the rat MSCs were then stained by fluorescent isothiocyanate (FITC) coupled or phycoerytrin (PE) conjugated mouse anti-rat antibodies (Abcam United Kingdom) and the human cells were stained with mouse anti-human antibodies (Abcam United Kingdom). The labeled cells were thoroughly washed with PBS and were analyzed on a flow cytometer (FACS Calibur Becton, Dickinson, United States), using WinMidi software (Scripps Research Institute; San Diego, United States). An isotype control with FITC or PE-labeled (Abcam United Kingdom) was included in each experiment and specific staining was measured from the cross point of the isotype with the specific antibody graph.
For osteogenic differentiation, the 4th passage cells were treated with osteogenic medium for three weeks with medium changes twice weekly. Osteogenic medium consisted of DMEM supplemented with 10-8 M/L dexamethasone (Sigma-Aldrich, St. Louis, United States), 10 mmol/L glycerol phosphate (Sigma-Aldrich, St. Louis, United States), 3.7 gr/L sodium bicarbonate (Sigma-Aldrich, St. Louis, United States) and 0.05 gr/L ascorbic acid (Sigma-Aldrich, St. Louis, United States). Osteogenesis was assessed by alizarin red staining.
To induce adipogenic differentiation, the 4th passage cells were treated with adipogenic medium for 3 wk. Medium changes were performed twice weekly. Adipogenic medium consisted of DMEM supplemented with 1 mol/L hydrocortisone (Sigma-Aldrich, St. Louis, United States), 0.05 gr/L ascorbic acid, 0.05 gr/L indomethacin (Sigma-Aldrich, St. Louis, United States) and 10-6 M/L dexamethasone. Adipogenesis was assessed by oil red staining.
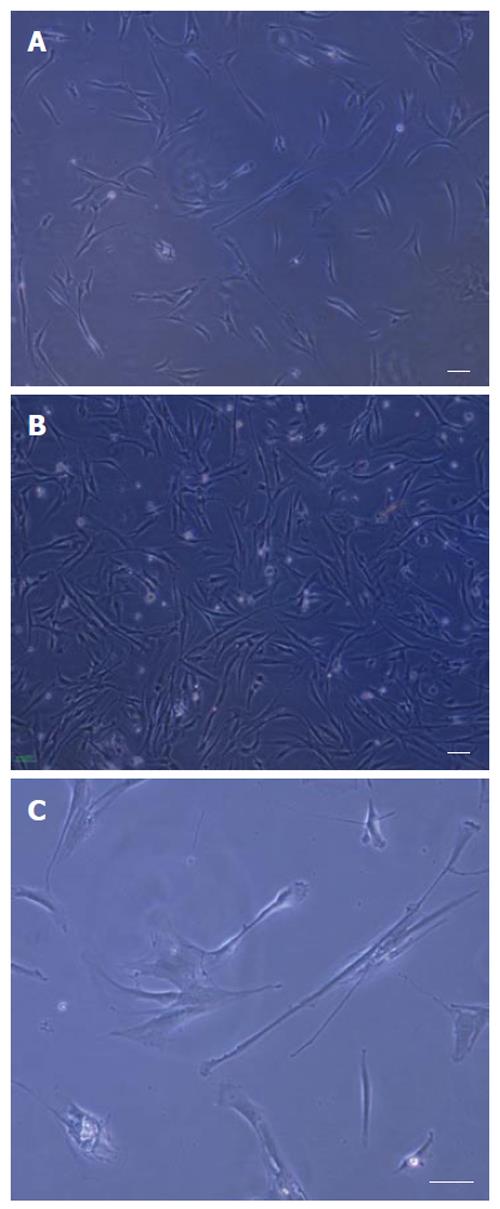
After the rat bone marrow-derived cells were isolated, the adherent cells were observed in the samples after 72 h culture. The proliferated cells were thin and spindle-shaped. Then, in the following 9-10 d, an adherent monolayer was achieved with a confluency of 50%-70% in all groups, which was the best time for the first passage (Figures 1A and B). Visual evaluation of the cultures of MSCs by phase contrast microscopy was used to demonstrate changes in the morphology with time in the culture and passage of the cells. As the culture proceeded, the cells had both slender and plump spindle-shaped morphology (Figure 1C). Later, in culture with passage, the large flat cells appeared on the rat MSC cultured in basal medium α-MEM and DMEM high glucose. Comparison between three concentrations of FCS (5%, 10% and 15%) showed that the cells gradually lose their proliferation capacity on DMEM low glucose containing 5% FCS (Table 1). The rat MSCs cultured in basic medium DMEM low glucose containing 10% and/or 15% FCS expanded rapidly for up to 10 passages.
| Time (wk) | Cell number per T25 culture plate | ||
| 5% FCS | 10% FCS | 15% FCS | |
| 2 | 6 × 105± 1% | 6 × 105± 1% | 6 × 105± 1% |
| 3 | 8 × 105± 1% | 1 × 106± 1% | 2 × 106± 1% |
| 4 | 1 × 106± 1% | 3 × 106± 1% | 4 × 106± 1% |
| 5 | 1.5 × 106± 1% | 6 × 106± 1% | 8 × 106± 1% |
| 6 | 1.8 × 106± 1% | 10 × 106± 1% | 12 × 106± 1% |
| 7 | 1.7 × 106± 1% | 13 × 106± 1% | 15 × 106± 1% |
The enrichment of the nucleated cells from red blood cells using Ficoll is an important step in the isolation of MSCs from the other cells in the bone marrow. The results obtained in the human mononuclear cells layer being rapidly removed from bone marrow was better in our selective method compared with the two previously reported methods[17,18].
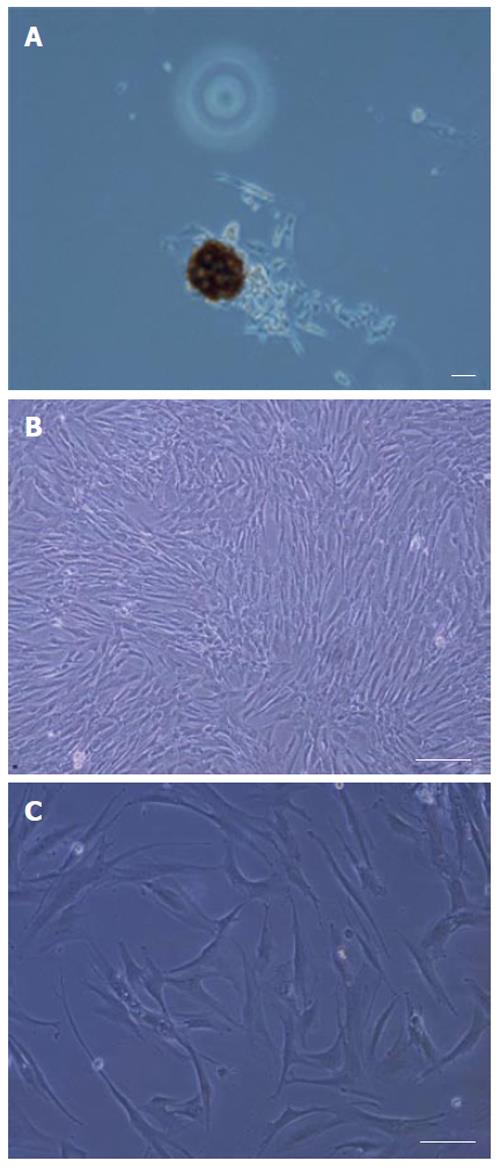
During culture, non-adherent cells were removed after 4 d by changing the medium. After one week, adherent cells were observed with heterogeneous compositions. By changing the medium twice a week and removing non-adherent cells, a relatively homogeneous culture was obtained after two weeks, composed of all which was morphologically similar to rat MSCs. The expansion of human MSCs in the culture was found to depend on the presence of colonies composed of a few fibroblast-like cells (Figure 2A). Bone marrow cells gradually generated a confluent layer of fibroblast like spindle-shape cells (Figure 2B). The first passage was performed after 12 d. However, there was no difference on the proliferation potential between the rat MSCs (on DMEM low glucose containing 10% FCS) and human MSCs after the second passage and the human MSCs expanded rapidly in culture. The cells increased in size and showed a polygonal morphology with evident filaments in the cytoplasm, especially when early passage cells were compared with late passage cells (Figure 2C).
At each passage, the cells were counted and analyzed for viability by trypan blue staining analysis, showing viability between 98% and 100% in all of the rat and human samples.
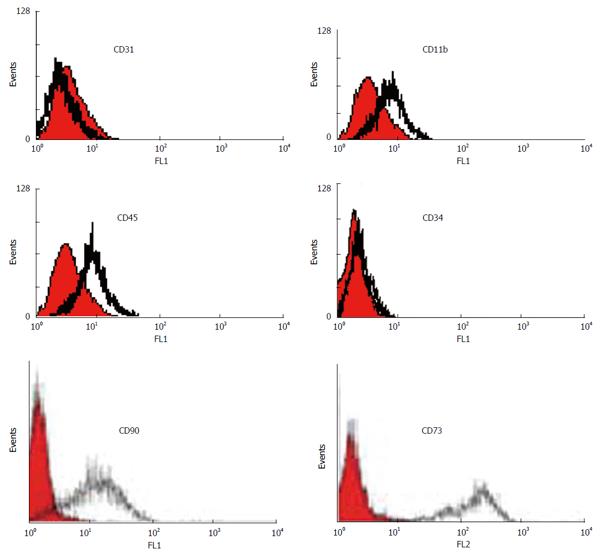
We characterized a population of cells resident within rat and human bone marrow cultures that expressed CD73 and CD90. Additionally, no cells expressed the hematopoietic markers CD45, CD34, CD11b (complement receptor) and endothelial marker CD31 [platelet/endothelial cell adhesion molecule (PECAM)-1] (data from the rat samples not shown) (Figure 3).
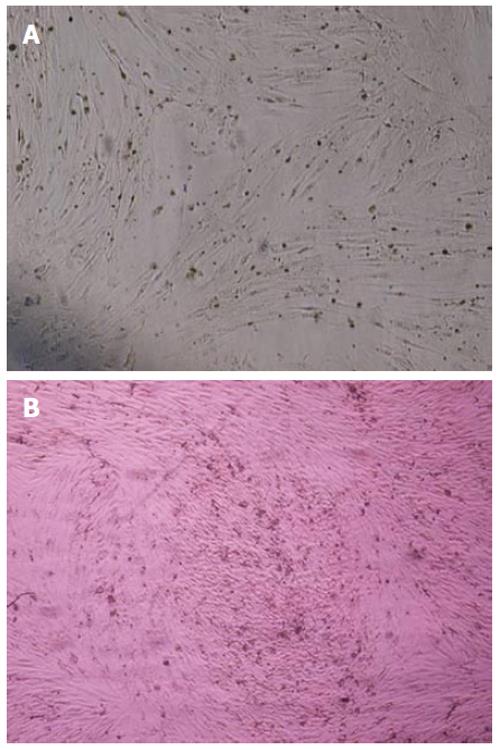
The MSCs derived from human and rat bone marrow samples were successfully characterized by differentiation potential into adipocytes and osteoblasts. To promote adipogenic differentiation, MSC cultures were incubated with adipogenic media. An accumulation of lipid-rich vacuoles within the cells was seen after 3 wk. Eventually, the lipid vacuoles combined and filled the cells. The differentiation of MSCs to osteoblasts in vitro involved incubating a confluent monolayer of MSCs with the osteogenic media for 2-3 wk. The MSCs formed aggregates or nodules and calcium accumulation could be seen over time. Lipid droplets in differentiated adipocytes were located by oil red O-staining. Likewise, alizarin red staining confirmed the presence of calcium deposits in osteocytes (Figure 2A and B) (data from the rat samples not shown).
The mesenchymal stem cell (MSC) is one of the most interesting types of the adult stem cell[20,21]. They have generated a great deal of excitement and promise as a potential source for cell-based therapeutic strategies, primarily owing to their intrinsic ability to self-renew and differentiate into functional cell types[22,23].
Despite diverse and growing information concerning MSCs and their use in cell-based strategies, the mechanisms that govern MSC self-renewal and multilineage differentiation are not well understood and remain an active area of investigation. Therefore, research efforts focused on biological and physiological characteristics of this highly useful stem cell type are crucial.
Due to the scarce number of human MSCs, the lack of universally accepted criteria on MSC-specific markers and their location of origin, more information concerning MSCs is derived from in vitro studies. For most of these experiments and trials, MSCs were expanded in the presence of FCS by their ability to adhere to plastic tissue culture dishes[8,9]. However, different variables and parameters must be considered in the isolation and expansion of MSCs for experimental and clinical use[13-15]. Moreover, cultures of human MSCs are morphologically heterogeneous; therefore, the establishment of homogeneous cell cultures and the development of efficient and particularly reproducible methods for the isolation and expansion of MSCs remains an important goal of this research field.
According to the above mentioned information, the main goal of this project was to improve isolation and expansion of human marrow-derived MSCs. As the rat MSCs or human-related cells are currently being tested in a number of animal models for human diseases and several clinical trials with these cells have been identified[24-26], we developed our experiments based on rat model samples.
To improve the isolation of human bone marrow cells, we compared three different methods according to previous studies[17,18] and our selective method, which was mentioned before. The results, in regard to obtaining human mononuclear cells more rapidly from bone marrow, were better when the cells were layered on Ficoll gradient, centrifuged at 2000 r/min for 30 min and then resuspended in DMEM with 5% FCS.
Despite the isolation of human MSCs from bone marrow, isolation of rat MSCs was not enhanced by using Ficoll gradient separation; indeed, a loss of MSCs was noted. Thus, proper dissection and dispersion of marrow from the long bones appear to be the key elements in red blood cell MSCs isolation, along with the use of low FCS concentration.
Tondreau et al[27] reported that isolation of bone marrow MSCs by negative selection using magnetic beads permitted a homogenous population of MSCs of more than 90% after 10 d of culture. In our experiment, similar homology was observed in morphology and flow cytometric analysis after 2-3 passages if the plastic adhesion was used as the selection method. Moreover, the cultures can be expanded and maintained for variable periods, depending on the species. For example, rat bone marrow derived stem cells expanded more rapidly than human stem cells after plating at the first passage. However, there was almost no difference between the proliferation potential from the rat and human MSCs at the third passage and the human MSCs expanded rapidly in culture.
After isolation and culture, the ideal culture conditions maintain MSCs with (1) phenotypic and functional characteristics similar to those exhibited in their original niche; (2) indefinite proliferation; and (3) a capacity to differentiate into multiple lineages[9]. Several studies were performed on the culture-composition related effect on the number, cellular growth and biological characteristics of the cells identified as MSC[8,17]. Some other studies reported that the culture parameters, including the tissue culture substrate or specific culture media, affect the final outcome[14,28]. In this way, we changed several parameters for culture of rat marrow-derived MSCs, including the type of basal media. For example, we used DMEM with high glucose, DMEM with low glucose and α-MEM media. At the first passage, there was no difference on fibroblastic morphology and the cell-proliferation capacity between these three different culture media. During the fifth to seventh passages, the cells lose their morphology and proliferation potential on DMEM high glucose and α-MEM media. However, the cells expanded rapidly for up to 10 passages on DMEM low glucose. Therefore, the selected medium for the proliferation and expansion of human MSCs was DMEM low glucose.
Another parameter which is important in cell culture medium composition is FCS, which causes potential hazards in clinical trials. As previously reported, a concentration of 10% to 20% of FCS is required for expansion of MSCs in culture[8,14]. Unfortunately, serum-free media have not yet been defined for the isolation and expansion of human MSCs and studies using serum-free media refer only to differentiation studies in vitro[29]. Currently, interest is growing in the use of autologous serum for MSC isolation and expansion. However, cell therapy requires large numbers of MSCs, which in turn necessitate large amounts of culture media and subsequently large volumes of peripheral blood. Besides, the use of pooled human serum cannot help to overcome this because the allogeneic serum results in MSC growth arrest and death[30]. In this study, we compared different concentrations of FCS (5%, 10% and 15%) for rapid expansion of rat MSCs in culture. Although the cells expanded more rapidly at 15% concentration of FCS at the first passage, there was almost no difference between 10% to 15% concentration of FCS during subsequent passages. However, proliferation of the cells was arrested at the third passage without change in the morphology and the differentiation capacity on 5% FCS (Table 1). Therefore, the 10% concentration of FCS in the culture medium composition was used for human MSCs.
Identification of MSCs is based on their morphology, immunophenotyping and functional characteristics. In this project, MSCs derived from human and rat bone marrow samples were successfully characterized by immunophenotyping and differentiation potential into osteoblasts and adipocytes. Analysis of the cell surface antigens of confluent monolayer cultures in our study revealed a highly homogenous population of cells expressing CD73 and CD90, initially identified as antigens specific for nonhematopoietic bone marrow progenitor cells and associated with the cells capable of differentiating along multiple mesenchymal lineages. Furthermore, flow cytometric analysis of the cells was negative for CD45, CD11b, CD34 and CD31, the common markers of hematopoietic stem cells indicating lack of any hematopoietic cell contamination (Figure 3). In addition to the identification of MSCs based on their morphological or phenotypic characteristics, we also introduced a system of culturing MSCs that supports and maintains the optimal differentiation potential during long term culture expansion. The marrow-derived MSCs differentiated into adipocytes and osteoblasts when they grew in specific culture conditions. Osteoblastic differentiation was demonstrated by the accumulation of a bone-like mineralized matrix and adipocytic differentiation was shown by the presence of cytoplasmic lipid accumulation (Figure 4).
This piece of evidence, together with fibroblastic morphology, clonogenic capacity of the cells, negative and positive surface markers and differentiation functions, allowed us to conclude that these cells were MSCs.
In this study, we improved isolation and expansion of human bone marrow derived MSCs based on the rat sample experiments which support and maintain the optimal characterization of human MSCs.
The authors would like to thank the Anatomy Department of Shiraz University of Medical Sciences, Dr. Talaei T, Dr. Mesbah SF, Dr. Aliabadi E and Mrs Leili Rohani for their valuable assistance.
Several studies were performed on the culture-composition related effect on the number, cellular growth and biological characteristics of the cells identified as mesenchymal stem cells (MSCs). Due to the scarce number of human MSCs, the lack of universally accepted criteria on MSC-specific markers and the lack of a uniform approach for rapid expansion of these cells among laboratories, establishing an optimal cell culture system for in vitro expansion of MSCs is of critical importance.
The research hotspot is to improve isolation and culture of human bone marrow-derived MSCs based on the rat sample, which has become an often-used model species for human disease.
The authors cultured and characterized MSCs from rat to set up the different protocols used in this study based on the following two main goals: (1) To improve isolation and expansion of human bone marrow derived MSCs. In this way, they used different protocols and culture conditions, including the type of basal media and the culture composition; and (2) To analyze the morphology, immunophenotype and differentiation potential of human and rat MSCs after developing a selective culture condition system.
This piece of evidence, together with fibroblastic morphology, clonogenic capacity of the cells, negative and positive surface markers and differentiation functions, support the optimal characterization of human MSCs for further experimental and clinical use.
In this study, the authors changed several parameters for culture of rat marrow-derived MSCs and used different protocols and culture conditions, including the type of basal media (DMEM with high glucose, DMEM with low glucose and α-MEM), as well as the culture composition and different concentrations of fetal calf serum.
The article seems to be a methodical paper and a thorough investigation of different protocols with important statements.
Peer reviewer: Mariann Gyöngyösi, MD, PhD, Department of Cardiology, Medical University of Vienna, Wahringer Gurtel 18-20, A-1090 Vienna, Austria
S- Editor Wang JL L- Editor Roemmele A E- Editor Zhang DN
| 1. | Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267-274. [PubMed] |
| 2. | Siniscalco D, Sullo N, Maione S, Rossi F, D'Agostino B. Stem cell therapy: the great promise in lung disease. Ther Adv Respir Dis. 2008;2:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, La Russa VF. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5:1571-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease. Gut. 2007;56:716-724. [PubMed] |
| 5. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2027] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 6. | Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 7. | Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 457] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;249-282. [PubMed] |
| 9. | Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. [PubMed] |
| 10. | Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 889] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 11. | Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 12. | Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 542] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 13. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1410] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 14. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1668] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 15. | Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 743] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 16. | Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1606-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, Madon E, Fagioli F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 391] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 19. | Ayatollahi M, Kabir Salmani M, Soleimani M, Geramizadeh B, Sanati MH, Gardaneh M, Tabei SZ. Expansion of human marrow derived mesenchymal stem cells and their transdifferentiation potential. IRCMJ. 2010;12:446-452. |
| 20. | Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215-230. [PubMed] |
| 21. | Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004;95:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121-133. [PubMed] |
| 23. | Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 467] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 24. | Mariotti V, Melissari E, Amar S, Conte A, Belmaker RH, Agam G, Pellegrini S. Effect of prolonged phenytoin administration on rat brain gene expression assessed by DNA microarrays. Exp Biol Med (. Maywood). 2010;235:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Eaton MJ, Wolfe SQ. Clinical feasibility for cell therapy using human neuronal cell line to treat neuropathic behavioral hypersensitivity following spinal cord injury in rats. J Rehabil Res Dev. 2009;46:145-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Grant S, Tran P, Zhang Q, Zou A, Dinh D, Jensen J, Zhou S, Kang X, Zachwieja J, Lippincott J. Discovery of a novel class of targeted kinase inhibitors that blocks protein kinase C signaling and ameliorates retinal vascular leakage in a diabetic rat model. Eur J Pharmacol. 2010;627:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Tondreau T, Lagneaux L, Dejeneffe M, Delforge A, Massy M, Mortier C, Bron D. Isolation of BM mesenchymal stem cells by plastic adhesion or negative selection: phenotype, proliferation kinetics and differentiation potential. Cytotherapy. 2004;6:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells. 2006;24:1409-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Tao H, Rao R, Ma DD. Cytokine-induced stable neuronal differentiation of human bone marrow mesenchymal stem cells in a serum/feeder cell-free condition. Dev Growth Differ. 2005;47:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |