Copyright
©2011 Baishideng Publishing Group Co.
World J Stem Cells. May 26, 2011; 3(5): 43-52
Published online May 26, 2011. doi: 10.4252/wjsc.v3.i5.43
Published online May 26, 2011. doi: 10.4252/wjsc.v3.i5.43
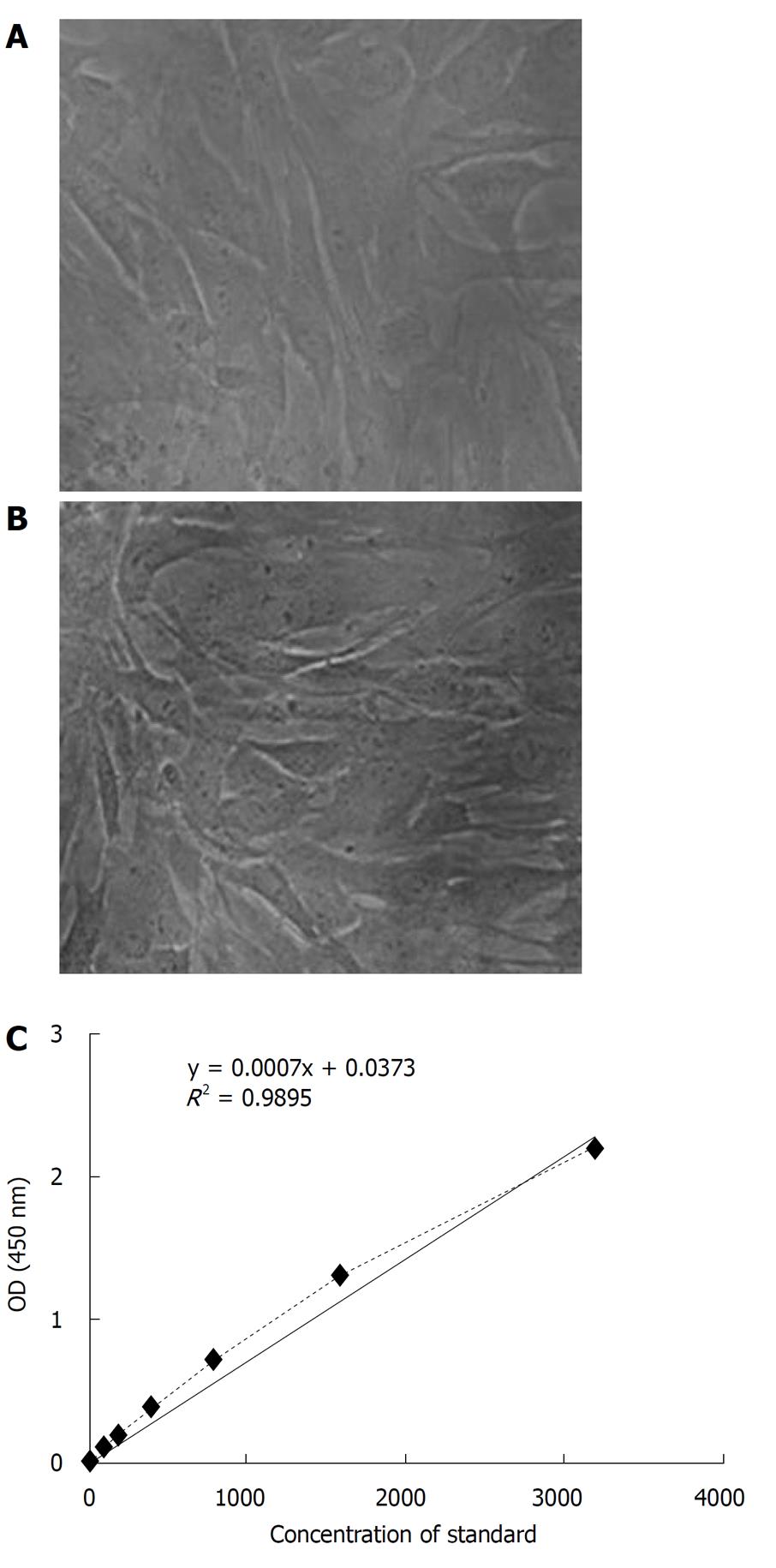
Figure 2 The determination of exogenous virus and stem cell factor residual in hematopoietic/progenitor stem cell product.
A: The cellular morphology of human fibroblasts cultured in α-MEM (× 400); B: The cellular morphology of human fibroblasts cultured in α-MEM with the cell suspension of hematopoietic/progenitor stem cell (HS/PC) product (× 400); C: The standard curve of residual stem cell factor (SCF) in the HS/PC product. It was detected by ELISA. After a curve (dotted line) was constructed by plotting the mean absorbance against the concentration, a best fitting curve (solid line) through the points on the graph was drawn.
- Citation: Guo CJ, Gao Y, Hou D, Shi DY, Tong XM, Shen D, Xi YM, Wang JF. Preclinical transplantation and safety of HS/PCs expanded from human umbilical cord blood. World J Stem Cells 2011; 3(5): 43-52
- URL: https://www.wjgnet.com/1948-0210/full/v3/i5/43.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v3.i5.43