Copyright
©The Author(s) 2025.
World J Stem Cells. Apr 26, 2025; 17(4): 102945
Published online Apr 26, 2025. doi: 10.4252/wjsc.v17.i4.102945
Published online Apr 26, 2025. doi: 10.4252/wjsc.v17.i4.102945
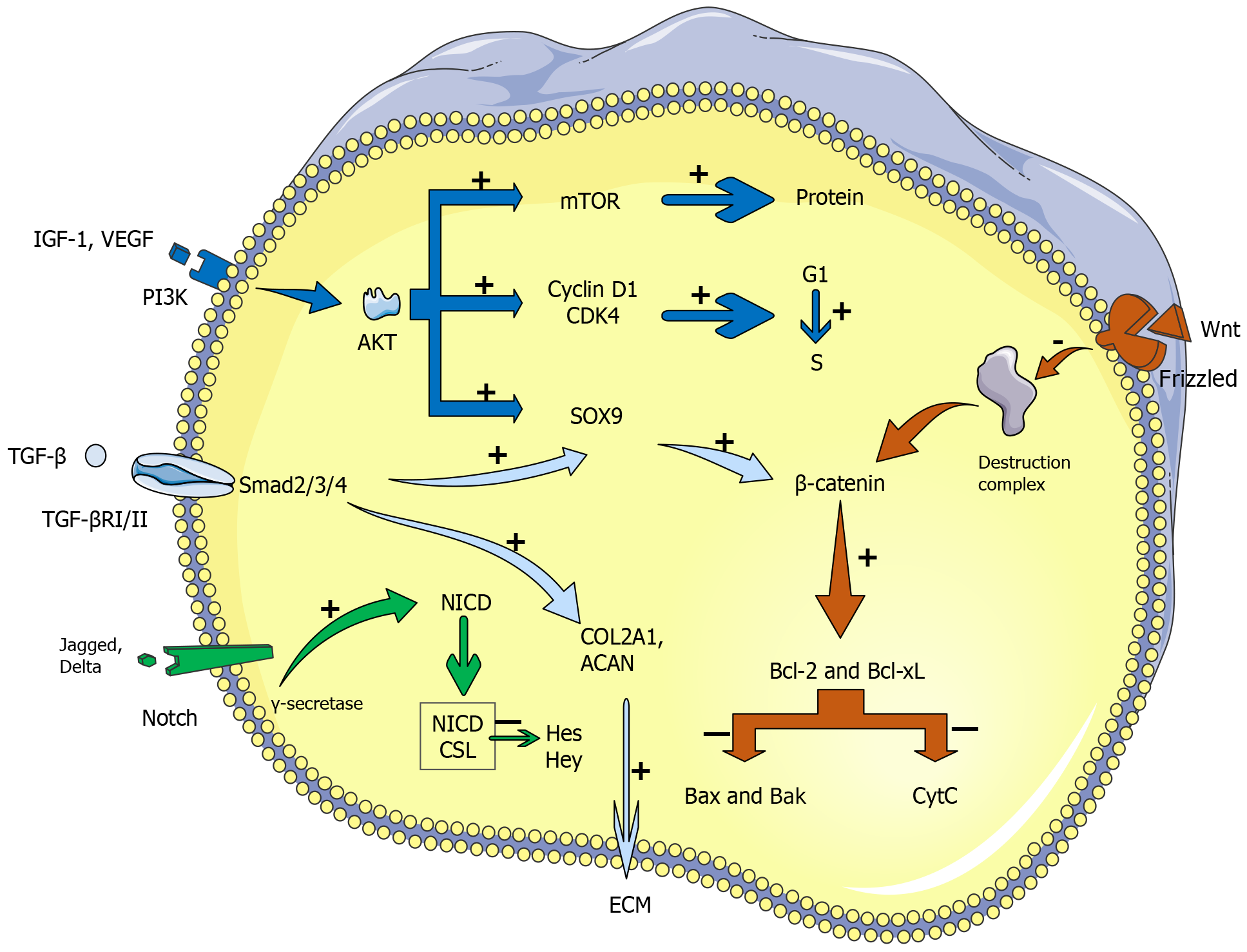
Figure 1 Main biological mechanisms of stem cell-mediated repair in damaged intervertebral discs.
Growth factors such as insulin-like growth factor 1 and vascular endothelial growth factor activate the phosphatidylinositol 3-kinase/protein kinase B pathway, which enhances protein synthesis through mechanistic target of rapamycin and promotes cell cycle progression via cyclin D1 and CDK4, facilitating stem cell proliferation. Transforming growth factor beta signaling, which is mediated by Smad2/3/4, promotes the expression of Sry-related HMG box 9, which is critical for chondrogenic differentiation, and the synthesis of key extracellular matrix components (COL2A1 and ACAN), which contribute to tissue repair. Notch signaling, which is activated by ligand binding (Jagged, Delta) to Notch receptors, leads to the formation of the Notch intracellular domain. The Notch intracellular domain downregulates Hes and Hey transcription factors, influencing stem cell differentiation. The Wnt/β-catenin pathway is pivotal for cell survival, regulating the balance between antiapoptotic (Bcl-2, Bcl-xL) and proapoptotic (Bax, Bak) factors to control apoptosis, thereby supporting the survival of stem cells in the damaged disc environment. These pathways work together to enhance tissue regeneration and restore intervertebral disc function. IGF: Insulin-like growth factor; VEGF: Vascular endothelial growth factor; PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; mTOR: Mechanistic target of rapamycin; SOX9: Sry-related HMG box 9; TGF: Transforming growth factor; NICD: Notch intracellular domain.
- Citation: Li ZP, Li H, Ruan YH, Wang P, Zhu MT, Fu WP, Wang RB, Tang XD, Zhang Q, Li SL, Yin H, Li CJ, Tian YG, Han RN, Wang YB, Zhang CJ. Stem cell therapy for intervertebral disc degeneration: Clinical progress with exosomes and gene vectors. World J Stem Cells 2025; 17(4): 102945
- URL: https://www.wjgnet.com/1948-0210/full/v17/i4/102945.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i4.102945