Copyright
©The Author(s) 2024.
World J Stem Cells. Jun 26, 2024; 16(6): 641-655
Published online Jun 26, 2024. doi: 10.4252/wjsc.v16.i6.641
Published online Jun 26, 2024. doi: 10.4252/wjsc.v16.i6.641
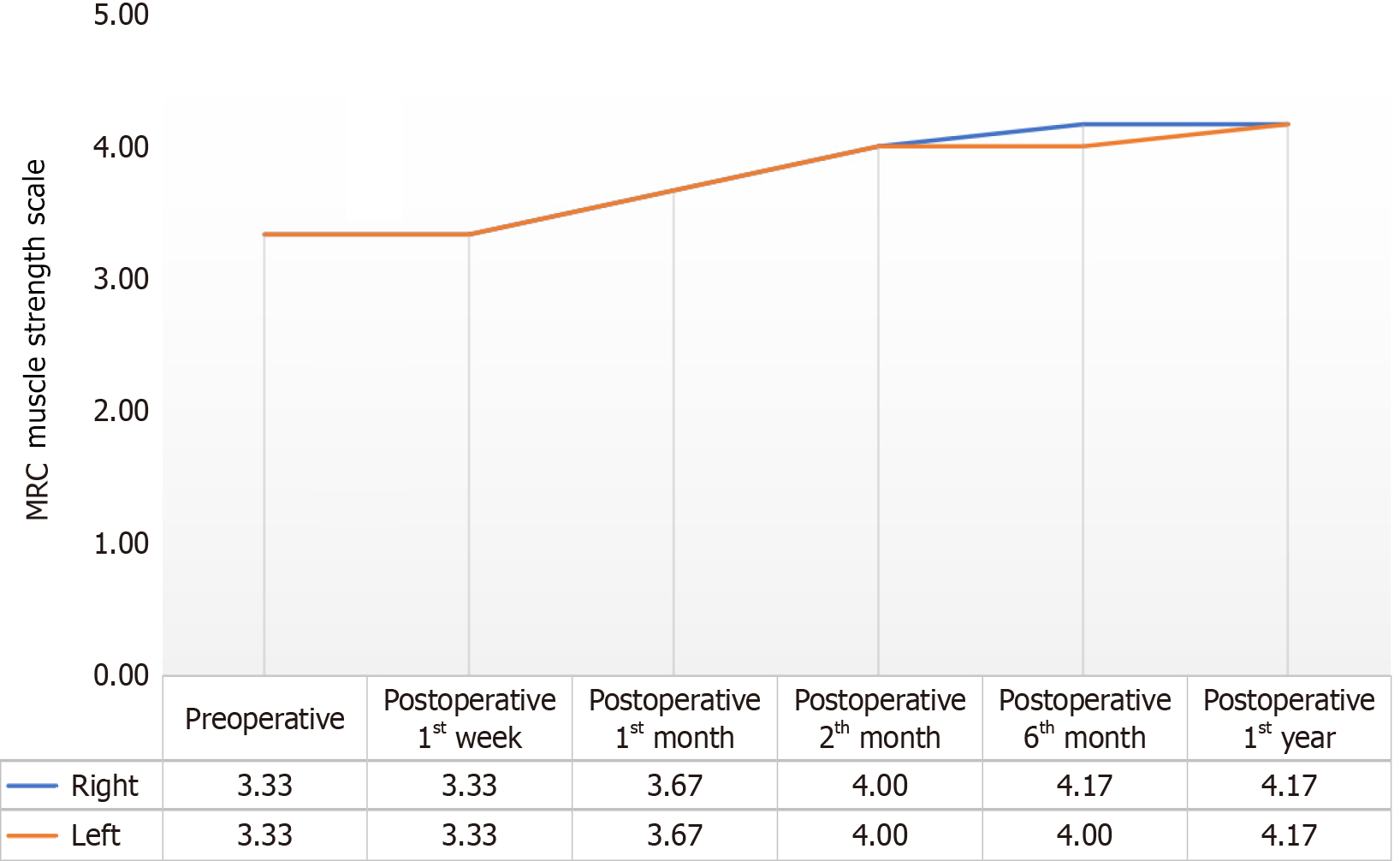
Figure 2 The changes observed in the average scores of the patients before the procedure, at the 1st wk, 1st month, 2nd month, 6th month and 1st year after the procedure, regarding the Medical Research Council muscle strength scale right and left values.
MRC: Medical Research Council.
- Citation: Kabatas S, Civelek E, Boyalı O, Sezen GB, Ozdemir O, Bahar-Ozdemir Y, Kaplan N, Savrunlu EC, Karaöz E. Safety and efficiency of Wharton’s Jelly-derived mesenchymal stem cell administration in patients with traumatic brain injury: First results of a phase I study. World J Stem Cells 2024; 16(6): 641-655
- URL: https://www.wjgnet.com/1948-0210/full/v16/i6/641.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i6.641