Copyright
©The Author(s) 2024.
World J Stem Cells. May 26, 2024; 16(5): 512-524
Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.512
Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.512
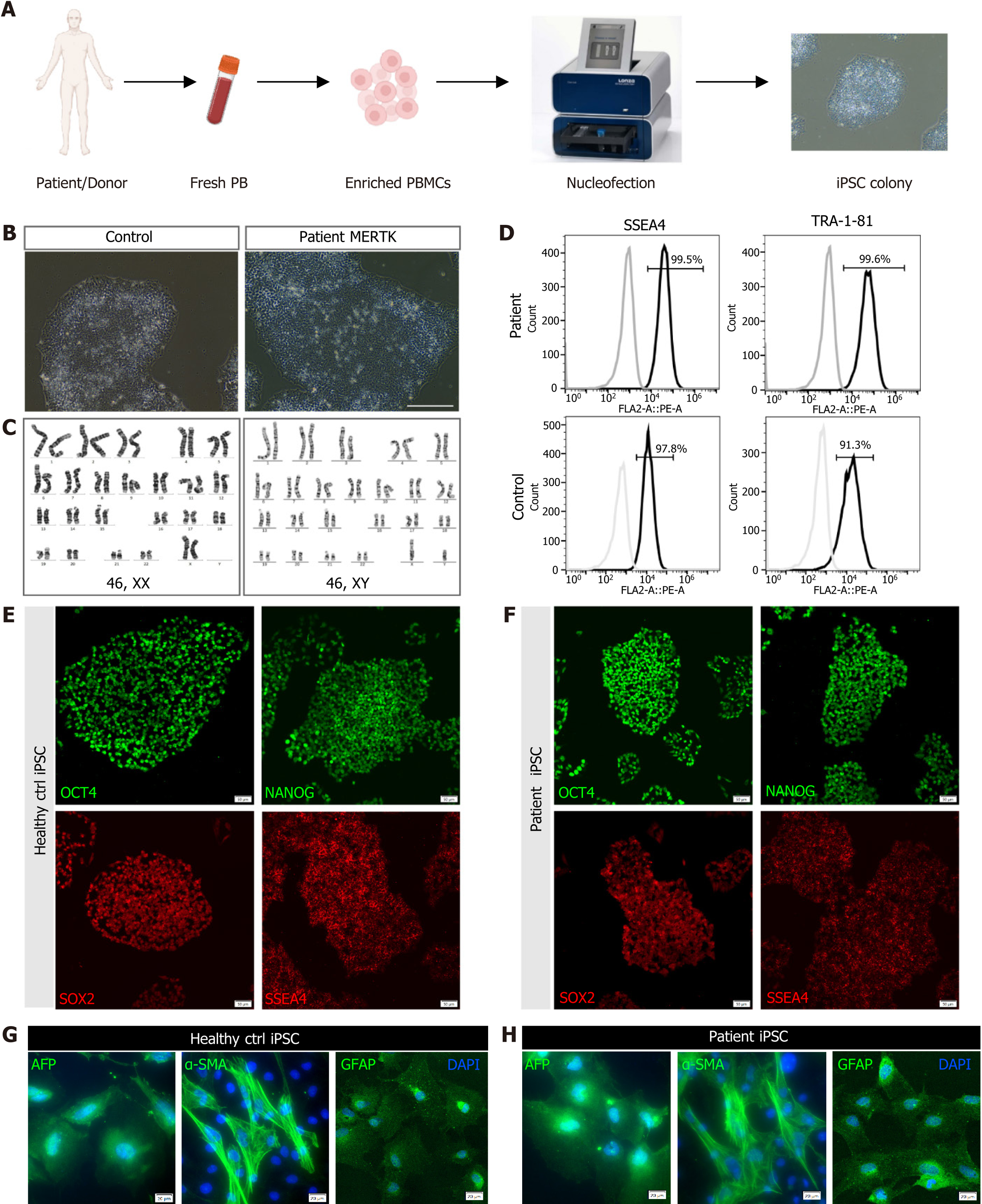
Figure 2 Generation and identification of the human induced pluripotent stem cells derived from the retinitis pigmentosa patient.
A: Timeline of human induced pluripotent stem cell generation; B: Imaging by phase-contrast microscopy. Scale bar = 200 μm; C: Karyotype analysis of the healthy control (left) and retinitis pigmentosa patient (right); D: Flow cytometry of pluripotency markers SSEA-4 and TRA-1-81; E and F: Immunostaining of pluripotency markers OCT4, NANOG, SOX2, and SSEA4 of the healthy control and retinitis pigmentosa patient. Scale bar = 20 μm; G and H: In vitro differentiation of control (G) and patient (H) induced pluripotent stem cells into three germ layers, endoderm (AFP+), mesoderm (α-SMA+) and ectoderm (GFAP+). Scale bar = 20 μm. PB: Peripheral blood; PBMC: Peripheral blood mononuclear cell; iPSC: Induced pluripotent stem cell.
- Citation: Zhang H, Wu LZ, Liu ZY, Jin ZB. Patient-derived induced pluripotent stem cells with a MERTK mutation exhibit cell junction abnormalities and aberrant cellular differentiation potential. World J Stem Cells 2024; 16(5): 512-524
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/512.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.512