Copyright
©The Author(s) 2024.
World J Stem Cells. Nov 26, 2024; 16(11): 944-955
Published online Nov 26, 2024. doi: 10.4252/wjsc.v16.i11.944
Published online Nov 26, 2024. doi: 10.4252/wjsc.v16.i11.944
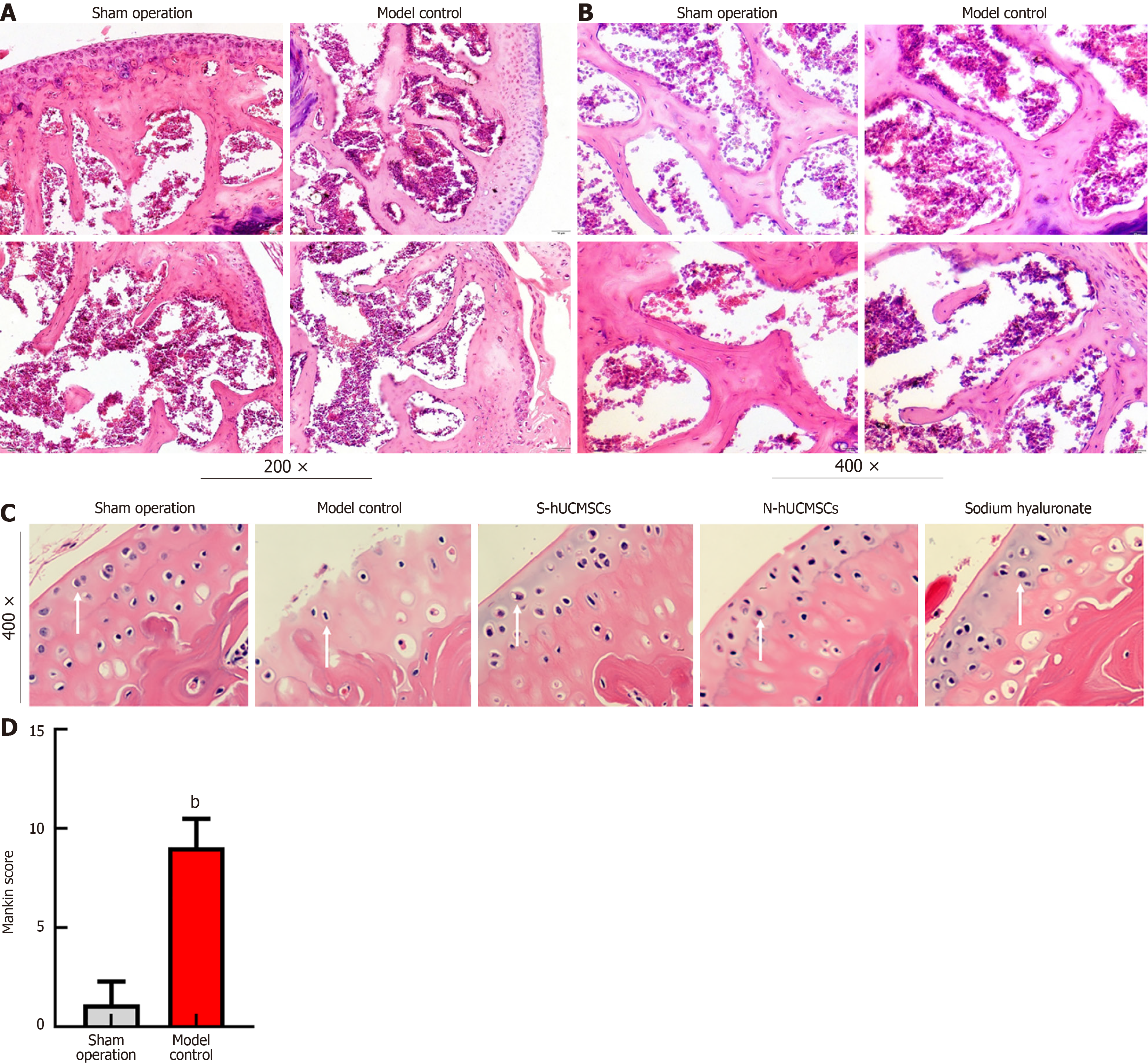
Figure 4 The experimental group favored the repair of cartilage tissue.
A and B: Model validation: In the control group, the integrity of the articular cartilage was disrupted, the cartilage surface became rough and uneven, the synovial tissue in the joint cavity proliferated significantly, and the number of osteoclasts in the bone marrow cavity increased; C: Experimental endpoints: The number of chondrocytes in the treatment group was significantly increased compared to the model control group; D: Experimental endpoints: The Mankin score of the model control group was significantly higher than that of the sham surgery group (blank control group). bP < 0.01, comparison with the sham-operated group. N-hUCMSCs: Serum-free human umbilical cord mesenchymal stems; S-hUCMSCs: Serum-cultured human umbilical cord mesenchymal stem cells.
- Citation: Xiao KZ, Liao G, Huang GY, Huang YL, Gu RH. Efficacy of serum-free cultured human umbilical cord mesenchymal stem cells in the treatment of knee osteoarthritis in mice. World J Stem Cells 2024; 16(11): 944-955
- URL: https://www.wjgnet.com/1948-0210/full/v16/i11/944.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i11.944